“Without a protective ozone layer in the atmosphere, animals and plants could not exist, at least upon land. It is therefore of the greatest importance to understand the processes that regulate the atmosphere’s ozone content.”
The Royal Academy of Sciences, announcing the Nobel Prize for Chemistry, 1995, for Paul Crutzen, Mario Molina and F. Sherwood Rowland.
Earth possesses an ozone layer, which can be considered as a “natural sunscreen” gifted by mother nature. It is vital for the survival of all life forms but often threatened mainly by many anthropogenic activities. This is acknowledged, such that on 16th September annually, World Ozone Day is celebrated. It spreads awareness in the community about the importance of the ozone layer, ozone depletion and traces out possible, suitable solutions to prevent it. This year 2021, is celebrated with the theme of “Montreal Protocol – Keeping us, our food and vaccines cool”. Let’s have an insight into some facts about the ozone layer, its importance, depletion, and suitable solutions from now on.
What is Ozone Layer?
Though called as “Ozone Layer”, this is not a typical layer, but a region in the lower stratosphere, approximately 15km to 35km from the earth’s surface, with a high concentration of Ozone (O3). Approximately 90% of the earth’s total ozone resides here. The thickness of the ozone layer varies with season and geographical location. It absorbs biologically harmful radiation, paving the way to successfully retain life on the earth.
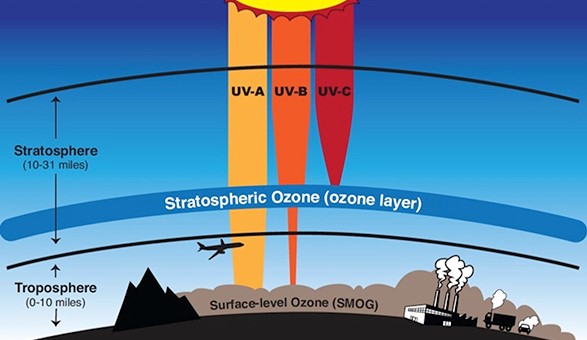
In the ozone layer, Oxygen (O2) and Ozone (O3) are naturally in equilibrium. Striking of ultraviolet radiation on oxygen molecules split them into two oxygen atoms, and one of that atomic oxygen combines with another oxygen molecule forming an ozone molecule. Under the influence of ultraviolet light, ozone again splits into an oxygen molecule and oxygen atom. This is called as “Ozone – Oxygen Cycle”.
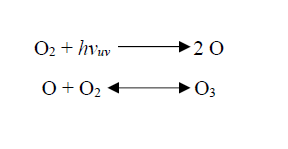
The ozone layer is capable of screening out harmful UV radiation. All UV-C radiation (280- 100 nm) is completely filtered out at an altitude of 35km. Almost all short wavelengths of UV-B (315- 280 nm) are entirely filtered out, but some amount of UV-B radiation with longer wavelengths reaches the earth’s surface, after screening. However, studies show that the ozone layer is transparent to most UV-A radiation (400- 315 nm). The layer has the maximum absorption at the wavelength of 290nm.
Why is it important?
All life forms on the earth are adapted to radiation, which is filtered by the Ozone Layer. UV-C radiation is extremely harmful to all living beings and ecosystems. Higher frequencies of UV-B radiation result in sunburns in mild exposure, cataracts, and immune system suppression. Excessive exposure to UV-A radiation results in genetic damage and skin cancer. Apart from that, excessive radiation exposure may cause premature aging of the skin, physical damage, and indirect genetic damage. All these adverse effects are blocked due to UV light screening of the ozone layer. This is also beneficial in slowing down global warming and climate change. In other words, the form and evolution of life on the earth would have been completely different without the ozone layer.
What is Ozone Depletion? What causes it? What are the impacts?
The term Ozone Depletion: the gradual thinning of the ozone layer due to gaseous compounds containing Chlorine or Bromine was first brought out into the light in 1969, by Dutch chemist Paul Crutzen, in terms of the Nitrogen Oxide catalytic cycle. Nowadays, ozone depletion is a widely discussed topic due to its massive impact on all organisms.
Causes for Ozone Depletion
Among the many causes which lead to ozone depletion, the release of Chlorine and Bromine from chemical compounds released from industrial and other sectors can be considered as the leading factor. Chlorine or Bromine atoms, released from Chlorofluorocarbons (CFC-11, CFC-12, CFC-13, CFC-114, CFC-115), Halons (Halon 1211, Halon 1301, Halon 2402), Carbon tetrachloride, 1,1,1-trichloroethane, Methyl bromide, Hydrochlorofluorocarbons (HCFCs), etc., combine with Ozone molecules to break them down, by releasing atomic Cl or Br as the outcome.
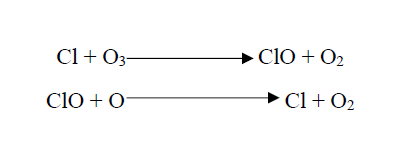
CFCs are commonly used as coolants in refrigerators, air conditioners, solvent cleaners, a blowing agent in the production of foam, and propellants in aerosols. Halons are often used as fire extinguishing agents. Carbon tetrachloride is used in the textile and electronic industries as a cleaning agent. 1,1,1-trichloroethane is used as a solvent for cleaning electronic circuit boards, in dry cleaning, and as a thinner in correction fluid. Their improved use since the last century, longer lifetime, and higher stability have caused a profound increase in ozone depletion. Apart from that, gaseous compounds released in a rocket launch also have some potential for ozone depletion. Due to the advancement of industries, the release of above Ozone Depleting Substances (ODS) has accelerated, such that ozone depletion was more profound in the last century.
However, some studies have shown that apart from the contribution of the Sun’s UV rays in breaking down ODS molecules and releasing Chlorine, cosmic rays are also responsible for breaking down those molecules.
The Ozone Hole
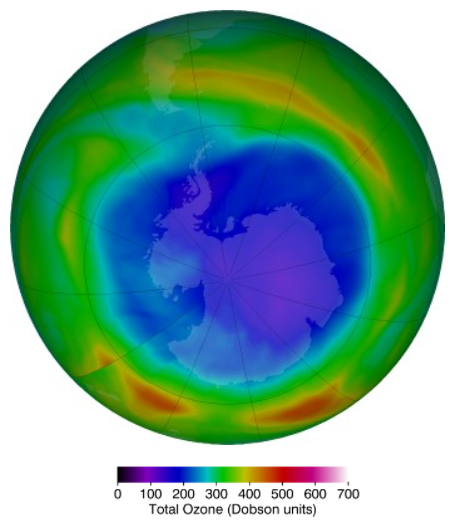
Ozone depletion is more severely observed in Polar Regions; especially in the Southern Pole. A severe drop of ozone level (even less than 220 Dobson Units) above the Antarctic, covering land larger than North America, was first reported in 1985 in springtime, which is known as “Ozone Hole”. However, after the realization of the hidden threat, due to the effective measures taken to minimize depletion, recent studies depict that the ozone layer has started to recover including Ozone Hole.
Impacts of Ozone Depletion
Without an ozone layer, we all would have been doomed. Ozone depletion has adverse effects on both human health and other ecosystems. Exposure to harmful UV radiation may cause an increased risk of eye diseases, skin cancers, and infectious diseases, due to suppressed immune systems. Especially, in light-skinned human populations, it will result in nonmelanoma skin cancer, as a result of impaired immune responses.
Harmful UV radiation may cause changes in species composition (mutations), plant form, and secondary metabolism resulting in altered biodiversity, implications in plant competitive balance, plant pathogens, and biogeochemical cycles. In aquatic ecosystems, phytoplankton production may reduce, and early developmental stages of fish, shrimp, crab, amphibians, and other mammals will also be affected. This may also cause a decrease in reproductive capacity and impaired larval development in aquatic ecosystems.
As mentioned, ozone depletion affects biogeochemical cycles. It results in changes in the production and decomposition of plant matter, primary production and release of important atmospheric gases, and reduction of bacterioplankton growth in Upper-Ocean, etc. Apart from that, due to the inhibition of nitrifying bacteria and photo decomposers, aquatic nitrogen cycling will be affected. Also, the marine Sulphur Cycle will be adversely affected.
Unfiltered UV-B radiation may result in higher photo disassociation of gases and may enhance the production of gases like Hydrogen peroxide (H2O2) which has adverse effects on human health. Also, altered hydroxyl concentrations in the atmosphere may alter the lifetime of gases like Methane and might substitute for CFS. Apart from that, harmful UV radiation may cause damages to polymers, both synthetic and natural, as UV-B rays accelerate biodegradation.
Although ozone depletion doesn’t have a direct impact on climate change, due to its potential in increasing the greenhouse effect, it may have an indirect impact on global warming and climate change.
What are the measures taken to reduce ozone depletion?
Due to the dangers associated, several global protocols and legislations were introduced to reduce ozone depletion, by minimizing and phasing out the consumption and release of ODSs. The first international action was taken in 1977 in Washington D.C with the participation of 32 countries.
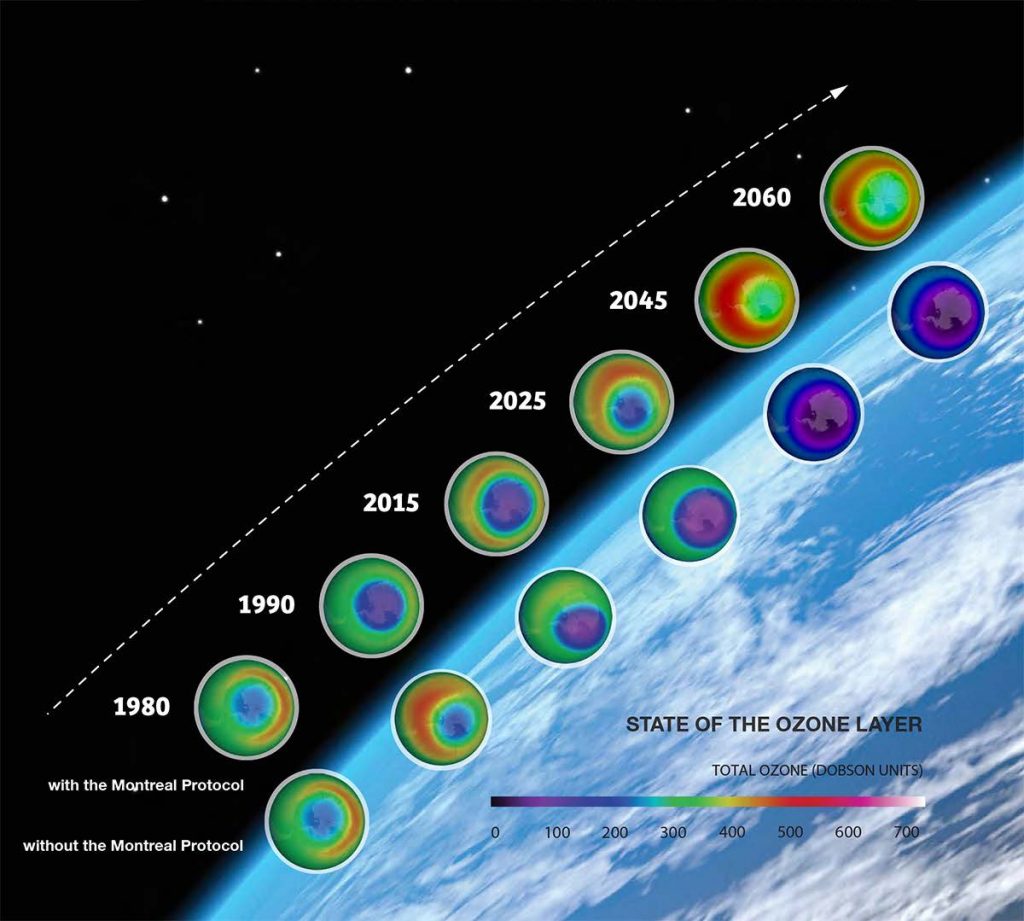
In 1985, Vienna Convention was established for the protection of the ozone layer. It can be considered as the first step towards the Montreal Protocol, which was signed in Montreal, Canada in 1987. The original protocol agreed for the complete phase-out usage of CFCs, Halons, carbon tetrachloride, and methyl chloroform. Several amendments were made from time to time, to increase its efficiency. Montreal Protocol can be considered as one of the most successful protocols ever implemented. Recent studies suggest that human-made chlorine and bromine will be completely removed from the stratosphere by the mid of 21st century, and the ozone layer will be recovered up to its state as it was in the 1980s.
Apart from them, several other treaties and legislation, such as the Australian Chlorofluorocarbon Management Strategy, Environmental Protection (ozone protection) Policy, United Nations Environment Programme, Ozone Protection, and Synthetic Greenhouse Gas Management Act 1989, The Ozone Action, etc. were implemented for the reduction of ozone depletion.
Sri Lanka has also signed for both the Vienna Convention and the Montreal Protocol. National Environmental Act, its amendments, and other related regulations regulate ozone depletion in Sri Lanka locally.
As a result of these legislations, substitutes for ODSs were introduced in several industries. For example, HCFC-22 was replaced by HFC-410A, and CFC-12 by HFC-134a. Alternative insulating materials, packaging materials such as plastic film bubble wrap, air conditioners, and refrigerators that operate on non-HCFC refrigerants, substitute food containers such as hydrocarbon-blown polystyrene, etc. have become an emerging trend in the modern world.
Moreover, an international day for the preservation of the Ozone Layer (World Ozone Day) is marked on 16th September since 1994, to increase awareness in the community. This year, it stresses the slowing down climate change and boosting energy efficiency in the cooling sector, which leads to food security, under the theme “Montreal Protocol – Keeping us, our food and vaccines cool”. It is directly relevant to the Kigali Amendment of the Montreal Protocol, which stressed the phasing out HFCs, which has lower potential in ozone depletion, but higher potential in global warming.
What can we do as responsible individuals?
The responsibility of protecting the ozone layer lies not only with global and government policies and institutions but also with each individual, who benefits from the screening process of the ozone layer. Using refrigerators and air conditioning machines that are free from HCFC as a refrigerant, consumption of aerosol products that do not use HCFCs or CFCs as propellants, proper inspection and maintenance of such machinery to prevent refrigerant leakage, and recycling of refrigerant in vehicle air conditioners can be considered as sensible and responsible acts of responsible citizens. Apart from personal usage, even in an industry, we, as responsible individuals should be careful when dealing with ODSs.
It is our utmost responsibility to protect the ozone layer, for the betterment of not only mankind but also of whole life on the earth. Let’s get on the zone, to save ozone starting from this World Ozone Day itself, for every moment in our lives, as individuals and as the whole global community who strive hard for a better world in the future.
Written By:
P.K.D. Chathumini Yasara,
1st Year Undergraduate,
Faculty of Science,
University of Colombo.
and
Shehani Rashmika Nawagamuwa,
1st Year Undergraduate,
Faculty of Science,
University of Colombo.
References:
- ozone layer | Description, Importance, & Facts. (n.d.). Retrieved September 10, 2021, from https://www.britannica.com/science/ozone-layer
- Ozone Layer Protection | Environmental Protection Department. (n.d.). Retrieved September 10, 2021, from https://www.epd.gov.hk/epd/english/environmentinhk/air/ozone_layer_protection/wn6_info_olp_ue_c.html
- T, S., & Reddy, K. K. (2011). Ozone Layer Depletion and Its Effects: A Review. International Journal of Environmental Science and Development, 2(1), 30–37. https://doi.org/10.7763/ijesd.2011.v2.93
- United Nations. (n.d.-a). International Day for the Preservation of the Ozone Layer. Retrieved September 10, 2021, from https://www.un.org/en/observances/ozone-day
- United Nations. (n.d.-b). Ozone Day | Ozone Science. Retrieved September 10, 2021, from https://www.un.org/en/observances/ozone-day/science
- United Nations Environment Programme & Ozone Secretariat. (n.d.). Montreal Protocol- Keeping us, Our food and Vaccines cool. Retrieved September 8, 2021, from https://ozone.unep.org/ozone-day/montreal-protocol-keeping-us-our-food-and-vaccines-cool
- United States Environmental protection Agency. (2021, August 30). International Treaties and Cooperation about the Protection of the Stratospheric Ozone Layer. Retrieved September 10, 2021, from https://www.epa.gov/ozone-layer-protection/international-treaties-and-cooperation-about-protection-stratospheric-ozone
- Wuebbles, D. (n.d.). ozone depletion | Facts, Effects, & Solutions. Retrieved September 10, 2021, from https://www.britannica.com/science/ozone-depletion
Image Courtesy:
- Title Image: https://bit.ly/2VK9S85
- 1st Content Image: https://bit.ly/3tLqKYp
- 2nd Content Image: https://go.nasa.gov/3zp9s4V
- 3rd Content Image: https://bit.ly/2YQR7Rq



2 Comments
H.B.J.Ashinka · September 17, 2021 at 2:03 pm
Great job. ❤ Keep it up.
Archana Rupasinghe · September 18, 2021 at 10:50 pm
Very inspiring thoughts environment sustainability and resourceful article.
Well done girls! 👍