Coronavirus has created a global level pandemic and potentially the biggest socio-economic downfall of the century. The virus namely, Severe Acute Respiratory Syndrome Corona Virus – 2 (SARS CoV-2) is suspected to has emerged during the end of 2019, from Wuhan, China. Marking an important zoonotic event it infected humans and began transmission from one to another creating the pandemic. It affected more than 90 million people across 219 countries. Also caused the death of more than 2.0 million patients since its emergence to date. This yet again proves the importance of a vaccine.
Being a member of the beta coronavirus group, SARS CoV-2 shares some similarities with SARS CoV and MERS viruses, such as using human Angiotensin-Converting Enzyme 2 (hACE2) of the host cell for the binding of the virus by both SARS CoV and SARS CoV-2. When the genetic sequence of SARS CoV-2 was first published on 11th January 2020, the attention of the whole world was drawn to find a vaccine against COVID-19. However, a successful vaccine needs a cautious validation of efficacy and side-effects. Thus various vaccines were started to develop on a variety of platforms including; virus vectored vaccines, monoclonal antibodies capable of providing passive immunity, genetic vaccines, etc. Each of these has its own advantages and drawbacks as well.
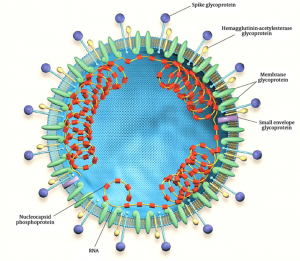
Coronavirus is a pleomorphic enveloped one with distinct projections of S proteins on the surface. It employs a positive sense single-strand RNA genome that is capped as well as polyadenylated. It complexes with nucleocapsid (N) proteins to form a helical nucleocapsid.
Immunotherapy is a highly practiced treatment against various pathological conditions including infectious diseases and cancers. Thus a lot of effort is put into developing a vaccine against COVID-19. The vaccine development landscape of COVID-19 is based on a few major technological platforms. They are; live attenuated virus, inactivated virus, non-replicating viral vector, replicating viral vector, recombinant proteins, peptide-based, virus-like particles, DNA-based, and RNA-based.
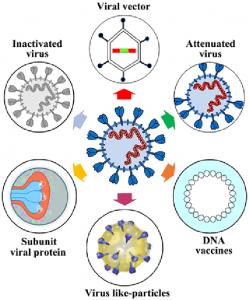
As expected, each of these platforms provides separate advantages and poses its own limitations. Live attenuated vaccines (LAV) uses the whole virus and has the potential to stimulate the immune system via toll-like receptors (TLR). They can be produced as derivatives from cold-adapted virus strains and by reverse genetics too. However, it demands extensive accessory testing to ensure efficacy. On the other hand, inactivated virus vaccines are rather stable and safe when compared with LAV. It has already been tested for various diseases including infection by SARS CoV where its immunogenicity can be uplifted using adjuvants. The drawback of the inactivated virus is that it needs booster shots to maintain immunity. Also during production large scale of virus amounts are required to be handled without compromising the integrity of immunogenic particles.
If protein subunits are used for vaccines, they would invoke fewer side effects due to lack of live viral particles when compared with others. Yet, it can induce an immune response and the memory for any future infections cannot be assured. Viral vector-based vaccines prevent the handling of infectious particles during development. It has in fact provided positive results with previous pandemics like MERS. Besides, it displays highly specific gene delivery to the host which triggers a vigorous immune response. The disadvantage is that, if the host has been previously exposed there would be immunity developed against the vector which causes the reduction of the efficacy of the vaccine. Besides, integration of the viral genome with that host may evoke adverse effects like cancers.
DNA-based vaccines have the advantage of being able to produce at accelerated rates, don’t need to handle infectious particles, and are quite temperature stable. However, it induces both cytotoxic and humoral responses within-host, might cause abnormalities in the host genome, and stimulate autoimmune conditions. RNA-based vaccines, pose no threat to the host genome by integration as the translation of mRNA takes place in the cytosol of the host cell. The problem with RNA-based vaccine is that it is rather unstable and show safety concerns regarding reactogenicity.
The effectiveness of a new vaccine is usually measured by its efficacy. For any disease, the development of a vaccine requires many steps assuring the safety and efficacy as well as the approval of regulatory agencies. The route of vaccine development can be simplified into 6 stages; Exploratory stage where basic laboratory researches are done, Pre-clinical stage at which animal testing is done to observe immune responses and to determine safe doses, Clinical development stage which consists of sub-phases as Phase 1, 2 and 3, Approval stage, Manufacturing stage where infrastructure, personnel, and equipment like factors are employed in mass scale, and Distribution stage at which proper management of vaccine is needed.
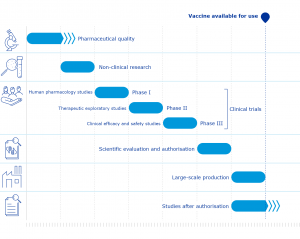
The clinical development stage plays a critical role in vaccine development. During its Phase 1 trial, the vaccine is given to healthy volunteers. Thereby the safety, as well as the dose, is confirmed. During the next trial of Phase 2, a small number of people with the targeted disease are vaccinated. Thus initial efficacy and explore the safety are observed further. Finally, at Phase 3 trials, large groups of patients are vaccinated to determine the full efficacy and safety.
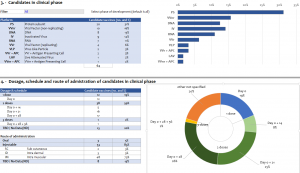
As of Tuesday, 19th January of 2020, according to the summary information on vaccine products mentioned in COVID-19 landscape of novel coronavirus candidate vaccine development worldwide provided by the World Health Organization, overall 64 vaccines are in the clinical development stage and 173 vaccines are in the pre-clinical developmental stage. Beijing Institute of Biotechnology in China, NIAID of USA, and Oxford University in the UK are a few of the leading researchers.
Based on the different platforms on which these vaccines are based, the most effective one will be the vaccines that could destroy the infected virus via antibodies providing passive immunity. This would provide immediate results in already infected patients and would be important to reduce the number of death. However, it would be most effective to use an attenuated virus to establish herd immunity by vaccinating healthy people before infection and develop immunity within them.
Unfortunately, developing a vaccine against COVID-19 has been a particularly difficult task due to some uncontrollable reasons. One such reason is that this particular virus is highly vulnerable to mutations. It undergoes an antigenic shift and antigenic drift as it spread from one population to another. These mutations vary according to environmental factors, geographic area, and population density too. Thus developers are straining themselves to find some consistency within these viruses. Meaning they are conserved and common in all strains so the vaccines could be effective regardless of the mutations. Also, storing and distribution of these vaccines demand much effort. Because most of the candidate vaccines require extreme cold temperature conditions to maintain integrity.
Developing a safe and at the same time highly effective vaccine against COVID-19 has been the need of the hour. If succeeded such a vaccine would induce immunity to terminate the pandemic. It is of global importance and priority to identify the international funding mechanisms to help the development, manufacturing, and distribution of vaccines. A pan-COVID vaccine is needed because the delay will cost hundreds of thousands of deaths. Yet, with appropriate funding and a good collaboration, the task would be feasible for the scientific community worldwide.
References :
- Thanh Le, T., Andreadakis, Z., Kumar, A., Gómez Román, R., Tollefsen, S., Saville, M., & Mayhew, S. (2020). The COVID-19 vaccine development landscape. Nature reviews. Drug discovery, 19(5), 305–306. https://doi.org/10.1038/d41573-020-00073-5
- Rab, S., Afjal, Javaid, M., Haleem, A., & Vaishya, R. (2020). An update on the global vaccine development for coronavirus. Diabetes & metabolic syndrome, 14(6), 2053–2055. Advance online publication. https://doi.org/10.1016/j.dsx.2020.10.023
- Kaur, S. P., & Gupta, V. (2020). COVID-19 Vaccine: A comprehensive status report. Virus research, 288, 198114. https://doi.org/10.1016/j.virusres.2020.198114
- https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
Image Courtesy :
- Title image: http://is.gd/lgLWuv
- 1st content image: http://t.ly/BG46
- 2nd content image: http://t.ly/Jaxu
- 3rd content image: http://t.ly/mhuD
- 4th content image: http://t.ly/Y6Ui
- 5th content image: http://is.gd/1lDIz7