Enzyme-Linked Immunosorbent Assay (ELISA) is a labeled immunoassay that is commonly used to detect as well as quantify antibodies, antigens, proteins, and glycoproteins in biological samples. It is of gold standard among immunoassays. ELISA is popular in the diagnosis of HIV infection, pregnancy tests, and blood typing, etc.
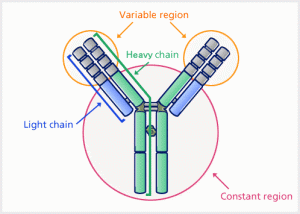
An antibody (Ab) is an immunoglobulin protein consist of two identical heavy chains and two identical light chains that recognize a particular epitope, on an antigen and facilitates the clearance of that antigen. An antigen (Ag) is any substance often foreign to the body, that binds specifically to an antibody or a T-cell receptor at the antigen-binding variable region. ELISA utilizes this interaction for the identification and quantification of antibodies and antigens.
ELISA is performed typically in 96-welled polystyrene plates. Materials include primary/secondary detection antibody, analyte/antigen, coating antibody/antigen, buffer, wash, and substrate/chromogen depending on the technique type. A primary detection antibody is a specific antibody that binds only to the protein of interest. Secondary detection antibody is a second antibody with an enzyme-conjugated to it, that binds to a primary antibody.
General steps of an ELISA
- Coating
- Blocking
- Detection
- Final read
During the first step, a layer of antigen or antibody is adsorbed onto the walls of wells in the ELISA plate. In the second step, a blocking buffer containing an unrelated protein is used to block any free, uncoated sites in the walls of the wells. As the third step, an enzyme-coated detection antibody is added. This detection antibody could be either a primary antibody or a secondary antibody. It depends on the type of ELISA performed.
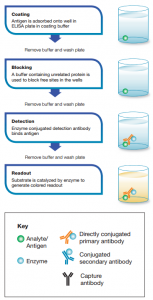
In the final step, a substrate capable of binding with the enzyme-linked to the detection antibody is added. Such a substrate is called a chromogenic substrate. Chromophore/chromogen/substrate generates color in the presence of the enzyme used for the detection. Color intensity is measured in form of optical density (OD). The most commonly used substrates are horseradish peroxidase (HRP) and alkaline phosphatase (ALP). The experiment uses a buffer like phosphate-buffered saline (PBS) for washing steps between each of the 4 steps to remove unbound excess free material. Specialized spectrophotometric plate readers are occupied to measure the absorbance of colored samples.

Types of ELISA
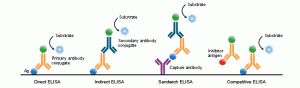
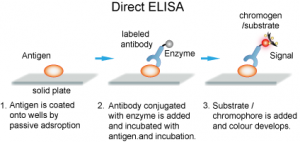
1. Direct ELISA
Direct ELISA immobilizes antigen in wells. A primary antibody conjugated directly to an enzyme binds and detects the antigen. This is the faster technique as it has few steps only. Thus it is less error-prone. However, its antigen immobilization is not specific. This technique is less flexible and has low sensitivity due to a lack of signal amplification. It is useful in analyzing immune responses for antigens.
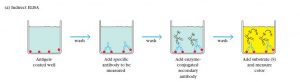
2. Indirect ELISA
The Indirect ELISA coats antigens in microtiter wells and employs serum samples containing primary antibodies specific for that particular antigen. They react to produce an antigen-antibody complex. Detection is a two-step process. A secondary anti-isotype antibody which is conjugated with an enzyme reacts with the antigen-antibody complex and binds with the primary antibody. Then substrate which is originally colorless reacts with the enzyme conjugated to a secondary antibody upon addition. This reaction generates a color.
Indirect ELISA has advantages like high sensitivity and great flexibility. The ability of more than one secondary antibody to bind the primary antibody and the ability to use different primary antibodies with a single labeled-antibody are the reasons for those advantages respectively. Yet, the possibility of cross-reaction of a secondary antibody and the need for many steps including incubation are the disadvantages. Indirect ELISA can determine the total antibody concentration of a sample.
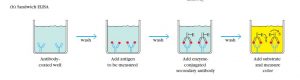
3. Sandwich ELISA
Sandwich ELISA uses matched antibody pairs as capture and detection antibodies. Each antibody is specific for a different and non-overlapping epitope of the antigen. Initially, this technique employs a capture antibody that binds the antigen. Then either direct or indirect ELISA configuration detects the bound antigen. There are many advantages of this type such as; very high specificity due to the involvement of two antibodies, sensitivity, and flexibility. But, the need to optimize the antibodies can be disadvantageous. Since samples are not necessary to be pure, complex samples use this method often.
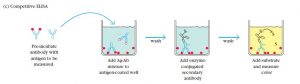
4. Competitive ELISA
Competitive/Inhibitive/Blocking ELISA is a rather complex method. Scientists predominantly use this method to measure the concentration of an antibody/antigen. In this method, the primary antibody is first incubated with the antigen sample that needs testing. The resulting antigen-antibody mixture joins another antigen that coats the microtiter wells. More antigens in the sample, fewer free antibodies in the mixture to bind the antigens coated in the wells. An enzyme-conjugated secondary antibody detects the primary antibody bound to well. Thus higher the antigen concentration in the original sample, weaker the output signal. The main advantage of Competitive ELISA is the lack of need to process the impure, crude sample. It is more robust and more consistent than other methods. This also accounts for the highest flexibility.
Types of data given by ELISA
- Quantitative
- Qualitative
- Semi-quantitative
The quantitative assessment draws a standard curve using the absorbance readings of serially diluted standard protein solutions with known corresponding concentrations. Thus for a sample of unknown antibody concentration, experimentally obtained absorbance readings can be extrapolated to find the relevant concentration using the standard curve and calculate the target protein concentration. The qualitative assessment compares positive or negative results upon the coloration and absorbance readings against the negative control to determine the presence or absence of a particular target protein in the tested serum sample. Semi-quantitative ELISA gives negative or positive results along with a comparison of the target protein levels in the assay samples.
References :
- Owen, Judith A., Punt, Jenni., Stranford, Sharon A., Jones, Patricia P.Kuby, Janis. (2013) Kuby immunology /New York: W.H. Freeman
- Aoyagi K, Ashihara Y, Kasahara Y. Immunoassay and immunochemistry. In: McPherson RA, Pincus MR, eds. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 23rd ed. St Louis, MO: Elsevier; 2017:chap 44.
- Alhajj M, Farhana A. Enzyme-Linked Immunosorbent Assay. [Updated 2020 Mar 27]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK555922/
Image Courtesy :
- Title image: http://is.gd/iOwBgp
- 1st content image: http://is.gd/vhHdUd
- 2nd content image: http://t.ly/if6F
- 3rd content image: http://t.ly/UpRV
- 4th content image: http://t.ly/N1yh
- 5th content image: http://t.ly/cKO2
- 6th content image: http://t.ly/5z1t
- 7th content image: http://t.ly/iLxJ
- 8th content image: http://t.ly/UOyn
- 9th content image: http://t.ly/PvuK

