Nowadays, we are concerning more about personal health hygiene. Among these precautions to prevent the spreading of coronavirus, one of the most important things is using hand sanitizers. The key advice has been to make sure that you regularly wash your hands to prevent spreading the disease. What is the scientific story behind this? Is it enough to use hand sanitizers to prevent this condition? Let us take a journey to investigate the hidden chemistry inside a hand sanitizer bottle and they use it out.
History of hand sanitizers
For the first time in history in the mid of 1800, a Hungarian doctor named Ignaz Semmelweiss discovered the link between handwashing and preventing the spreading of diseases. At that time doctors used soap and water for handwashing to prevent skin contaminations. But later in 1966 Lupe Hernandez, a nursing student in California invented the first-hand sanitizer using an alcohol and a gel combination. By 1965, the world’s first marketable hand sanitizer was on the shelves of supermarkets all over Europe. The scientists tried to improve the quality of the product using different proportions of chemical compounds.
The chemistry of hand sanitizers
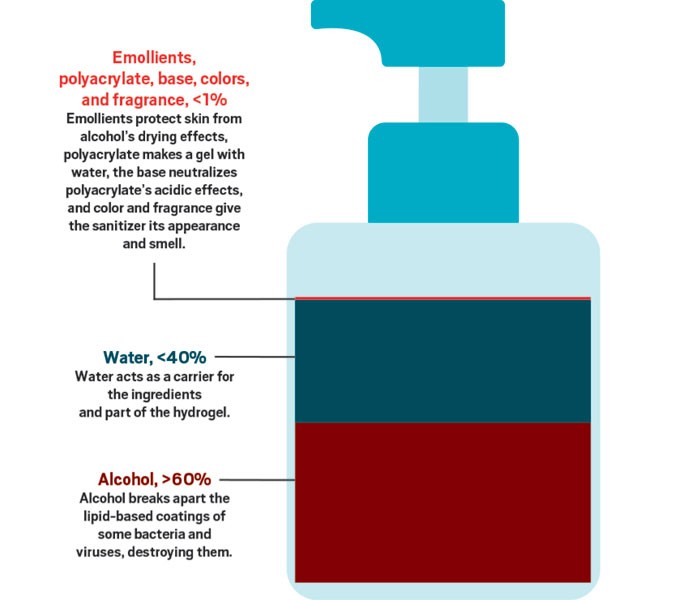
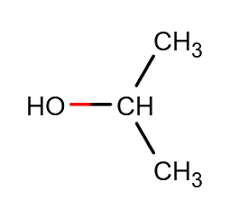
There are two types of hand sanitizers based on the ingredients. They are alcohol-based and non-alcohol-based sanitizers. We all know that alcohols have the superpower to destroy disease-causing agents. According to the Clinical Microbiology Review published in 2014, alcohols destroy the microbes by denaturation of proteins, cell splitting, and messing with cell metabolism. The wonder of the alcohol is the effectiveness of the disinfectant property increases with the concentration of the alcohol. The maximum effectiveness can be obtained by using a 90-95% alcohol concentration. Beyond that concentration, effect is less because for the protein denaturation there should be water molecules surrounding them. We know that bacteria have the ability to resist a certain compound if the particular compound is used for a long period of time. But alcohol does not lose its effectiveness with continued use. Due to these properties of Alcohol, they are used in hand sanitizers. These alcohol-based sanitizers usually contain ethanol, isopropanol (rubbing alcohol), or propanol. This is because of their high solubility in water. But yet alcohols do not have the ability to destroy non enveloped viruses. So, what about the coronavirus? Yes, fortunately, coronavirus is an enveloped virus that can be destroyed by alcohol. As mentioned earlier, to get the maximum effectiveness as much as possible a concentration of 60-95% alcohol is used in hand sanitizers. Alcohol-free hand sanitizers contain quaternary ammonium compounds such as benzalkonium chloride. But Centre for Disease prevention and control states that benzalkonium chloride is not effective against coronavirus.
Apart from that additional ingredients are added in order to improve the quality of the product. Either Chlorohexidine or Benzalkonium chloride is added to both alcohol and non-alcohol-based sanitizers since these compounds are active against viruses. To prevent bacterial contamination the manufacturers, add Hydrogen peroxide and to give a moisturizing effect to the product glycerol is added. So always check the ingredient list before buying a hand sanitizer. Specially concern about the percentage of alcohol used in the manufacturing process.

How to use it?
According to the CDC soap and water are more effective than hand sanitizers at removing certain kinds of germs such as norovirus, Cryptosporidium, and Chloridoides difficile as well as chemicals. Apart from that sanitizers may not remove chemicals such as pesticides and heavy metals like Pb. According to Daniel Pastula, a neuro-infectious disease expert and neurohospitalist at the University of Colorado Hospital soap and water work better against coronavirus. This is because soaps have an amazing ability to disrupts the sticky bonds and slide off the virus. If soap and water are not readily available, it is better to use a hand sanitizer which contains at least 60% alcohol. But always try to use soap and water to clean your hands if they are visibly dirty or greasy. In that case, the grease or dirt will act as a protective coating to microbes. So, the method of cleaning hands depends on the cause of sickness.
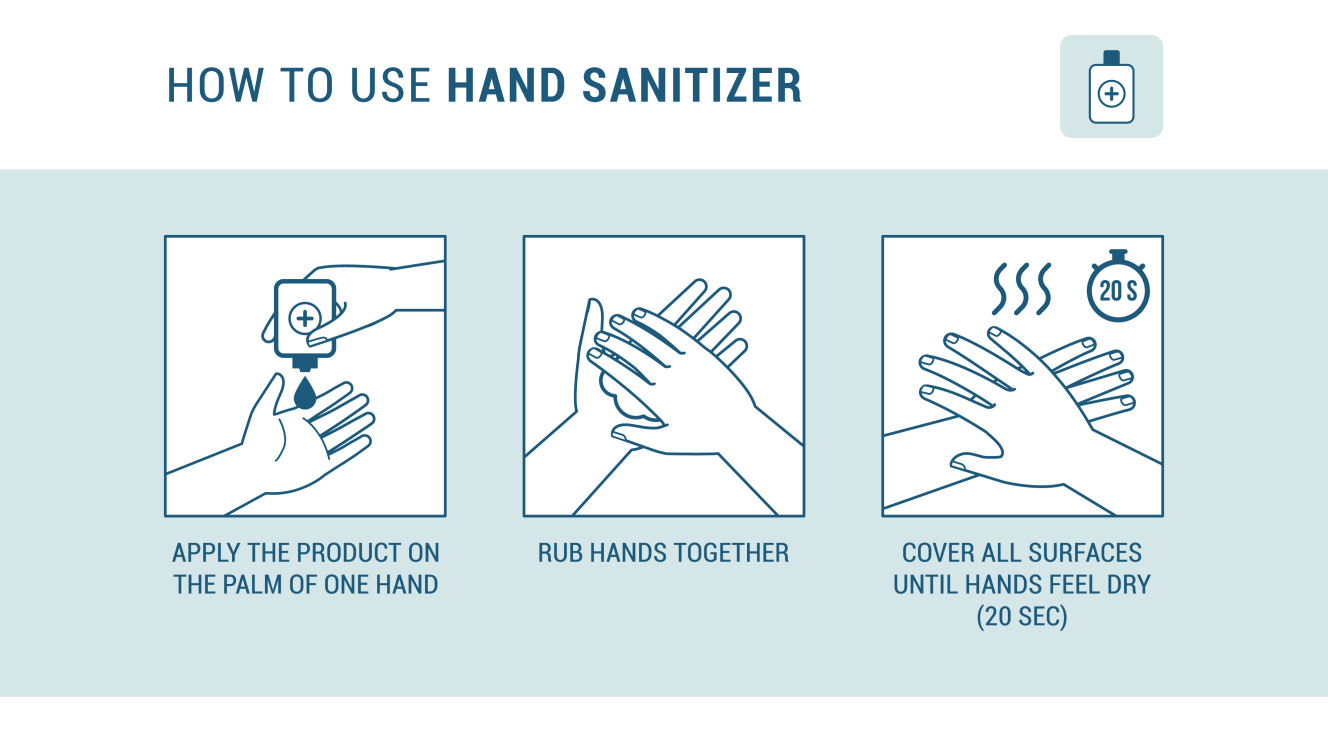
The correct method to use a sanitizer is as follows,
- First, apply enough sanitizer to your hands to cover all surfaces. Remember the volume of sanitizer is a much important factor. To properly coat the hands without leaving space to linger microbes use about 3ml of sanitizer.
- Then rub the hands together at least for 20 seconds until they become dry.
(Never rinse or wipe off the hands before the sanitizing liquid dry.)
Now it is the time to check whether your hand sanitizer bottle has completed the necessary requirements to act as an effective disinfectant against the coronavirus! In the next article let’s look deep into the chemistry behind soap and water.
Image Credits
- Featured image- https://bit.ly/324h0fv
- image 1-https://bit.ly/37YQ5p9
- image 2-https://bit.ly/3oNlJvO
- image 3-https://bit.ly/3jLEWKs
- image 4-https://bit.ly/3oL1y1j
References-
- https://www.cdc.gov/handwashing/hand-sanitizer-use.html
- https://www.livescience.com/hand-sanitizer.html
- https://www.britannica.com/topic/hand-sanitizer
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7301780/