Beep! The reading was “60”. Probably a mistake because the young boy stood there firmly. But within minutes, he collapsed with a cardiac arrest. The reading “60”, wasn’t his pulse, but the level of Oxygen saturation. With the ongoing COVID-19 pandemic, the “Pulse Oximeter” has become one of the essential instruments, even at your homestay. Sometimes, it may be the only instrument to make you aware, when to seek care before it’s too late.
Oxygen Saturation
Hemoglobin (Hb) is the main way that carries oxygen in our blood. Imagine an Oxygen molecule to be a person, Hb molecules to be boats, and the rivers on which they travel, to be the blood vessels. A single boat has a maximum occupancy of 4 people, which is analogous to a single hemoglobin molecule that can bind four oxygen molecules.

In a situation where 5 boats out of 10 are completely occupied with people, the Oxygen saturation is 50%. If 7 boats are fully occupied, it will be 70%.
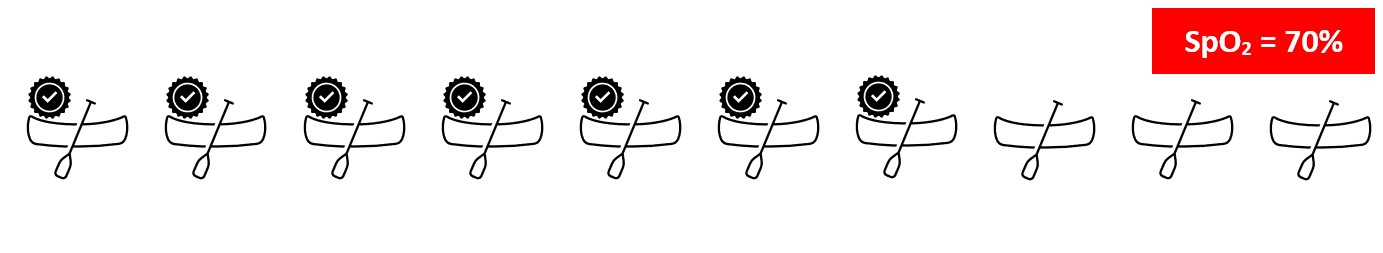
Similarly, if all 10 boats are occupied with people, the oxygen saturation is 100%. In short, oxygen saturation gives the percentage of the total hemoglobin that is carrying oxygen in the blood.
How does a Pulse Oximeter work?
Pulse oximetry incorporates light to determine the oxygen saturation in the blood (Indicated as SpO2) along with the pulse. Simply it consists of a light source that emits red light and infra-red light, a detector, and an electronic setup including a display to show the relevant details.

When a finger is inserted into the gap, as shown above, the amount of light received by the detector varies, as a certain amount of light is absorbed by the finger. This depends on some physical properties which are used by the instrument to determine the levels of oxygen saturation.
The amount of light absorbed depends on,
- The concentration of the light-absorbing sample
- The path length of light or thickness of the sample
- Selective absorption of Oxy Hb and Deoxy Hb
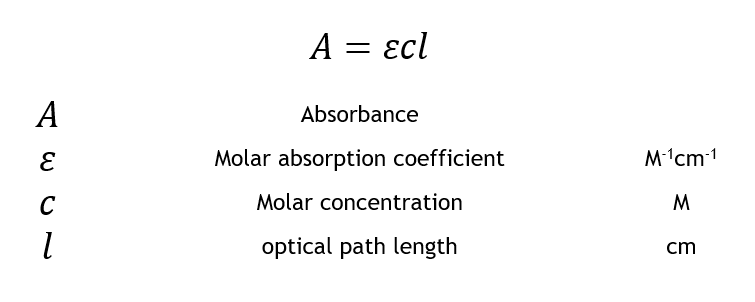
Points 1 and 2 are expressed in Beers’s Law and Lambert’s Law of Absorption respectively. Beer’s Law states that the degree of absorption is directly proportional to the concentration of the sample. Lambert’s law states that the degree of absorption is directly proportional to the thickness (length of the path that light travels) of the sample. Considering both together, the amount of light absorbed in the Pulse Oximeter is directly proportional to the concentration of Hemoglobin within the area of interest and to the length at which the light passes (arterial thickness).
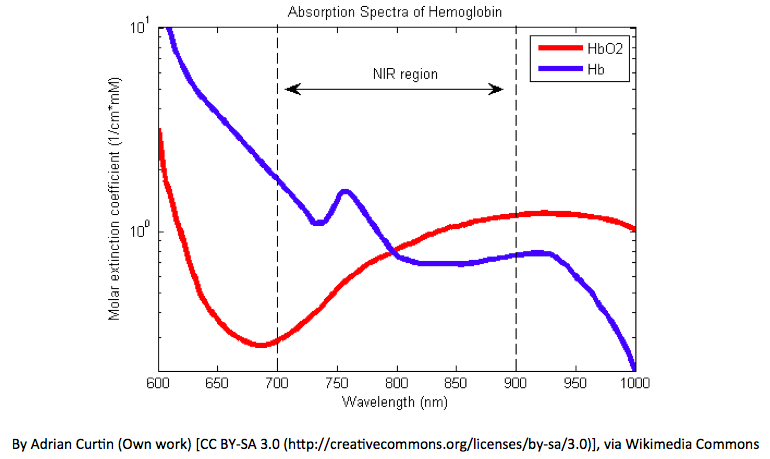
Apart from these, Hemoglobin absorbs different amounts of light at different wavelengths. Oxyhemoglobin absorbs a greater amount of infrared at 950 nm than red light at around 650 nm. Whereas Deoxy hemoglobin absorbs a greater amount of red light than IR. This is practically evident, as well-oxygenated blood will appear bright red color whereas deoxygenated blood will appear much darker, due to the absorption of more red light.
The ratio of red light absorbed compared to the amount of infrared light absorbed, changes depending on the amounts of oxy Hb and deoxy Hb present within the area of interest. The change of absorbance with the wavelength of light for Oxy Hb and Deoxy Hb is shown below. Taking the ratio of the relative absorptions into account, the oxygen saturation can be monitored accordingly.
A calibration graph is necessary to compensate for the errors that may arise when applying Beer-Lambert’s law directly to calculate the oxygen saturation. The calibration graph is plotted with the blood taken from volunteers between oxygen saturation limits of 100% to 75%. Any saturation below that limit is not accounted for as it would harm the volunteers. Hence for saturations below 75%, the pulse oximeters give less accurate readings.
A doubt may arise whether the accuracy of the reading depends on the thickness of the finger. The skin and other tissues too absorb some amount of light. Hence the thickness of the finger will be an issue considering that the pulse oximeter should account for only the light absorbed by arterial blood.
To overcome this issue, pulse oximeter analyses only pulsating blood. Since arterial blood being the only pulsating site in the finger, the rest can be cut off as non-pulsating. Since arterial blood is pulsating, the absorption of light observed changes with time. The light absorbed by non-arterial tissues remains constant with time, as they are considered non-pulsating. Hence both the total absorption and non-changing absorption are calculated. Then the non-changing absorption is subtracted from the total to obtain the changing absorbance.
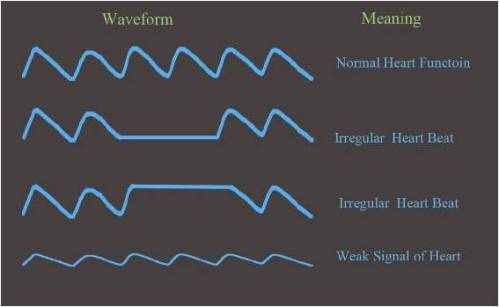
The graph of absorption vs time is called a Plethysmograph (Pleth). In sophisticated pulse oximeters, the Pleth is shown in the display, indicating how the pulse varies with time. It is vital to first observe the Pleth before looking at the oxygen saturation, as an abnormal pulsatile signal than the norm would give rise to errors when calculating the oxygen saturation.
It is important to account for ambient (room) lighting as it is considered as an unwanted light in pulse oximetry measurements. This too should be cut off by subtracting the ambient light absorption from the total relative absorptions of red, infra-red, and ambient lighting at a particular instance.
Limitations and Problems
Moving the finger within the pulse oximeter while taking a reading will lead to errors in calculating the oxygen saturation. Even the simple fingernail polish applied can affect the accuracy. Too much ambient lighting can give rise to errors. Hence it is advisable to be away from strong room lighting while a reading is taken. Like any instrument, it is not to be kept near high external electromagnetic radiation which will give errors in the reading. Furthermore, it is vital to adhere to the relevant environmental parameters concerning storage.
The oxygen saturation is limited to 100%. That is Oxygen dissolved in blood plasma will not be accounted as extra oxygen. That is a condition called hyperoxia, where excess oxygen becoming harmful, cannot be detected.
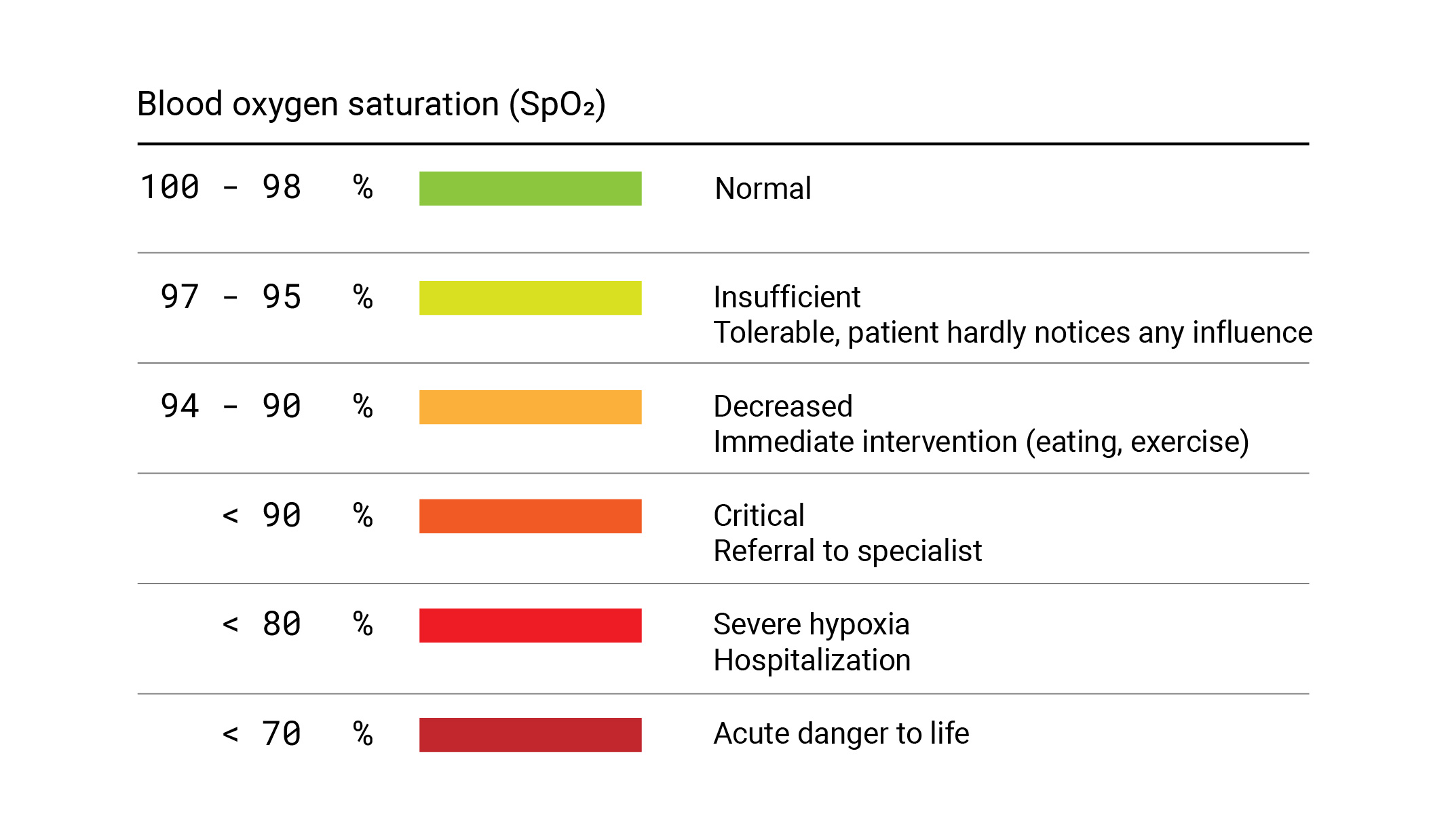
“Silent Hypoxia” is one of the main causes of unnoticeable deaths of COVID-19 patients, where the oxygen levels of blood can drop down without the patient noticing it. In this crisis, the Pulse Oximeter, being that simple gadget, has been a potential lifesaving solution.
Image Credits:
- Featured image: https://bit.ly/3ko6iZy
- Figure 1: https://bit.ly/38mg8FL
- Figure 2: Author
- Figure 3: https://bit.ly/3yl275O
- Figure 4: https://bit.ly/3zyIfNH
- Figure 5: https://bit.ly/38gsjUD
- Figure 6: https://bit.ly/2WpHEQq
- Figure 7: https://bit.ly/3BeWlo0
References:
- https://www.thoracic.org/patients/patient-resources/resources/pulse-oximetry.pdf
- https://www.howequipmentworks.com/pulse_oximeter/
- https://medicine.uiowa.edu/iowaprotocols/pulse-oximetry-basic-principles-and-interpretation

