The drug “Thalidomide” was created and manufactured by a German pharmaceutical company, the Grünenthal Group in the early 1950s. Thalidomide was famous for its effective treatment of morning sickness in pregnant mothers and was widely used during the late 1950s and early 1960s. However, after a few years of widespread use of the drug in Europe, Australia and Japan, it was found that thalidomide treatment resulted in approximately 10,000 babies born with deformities of the limbs, a disorder known as Phocomelia. In addition to abnormalities of the limb, the other effects attributed to the drug were congenital heart disease, malformations of the inner and outer ear and ocular abnormalities (Miller and Strömland, 1999). Despite the ban on thalidomide, some countries continued to provide access to the drug (Lenz, 1988) and despite its negative effects on infants, it was proven to be a useful treatment for leprosy and multiple myeloma (cancer of plasma cells).
The disastrous effect of this drug had a vast theory behind it, a theory which was studied intensely only after this tragedy had occurred. The chemical compound in thalidomide, α-glutarimide, had two forms known as “enantiomers” which are almost absolutely identical except that they were mirror images of each other which could not be superimposed. The general structure of the compound is as follows:

Glutarimide contains a single asymmetric carbon and therefore may exist in two optically active forms (enantiomers) designated as R and S.
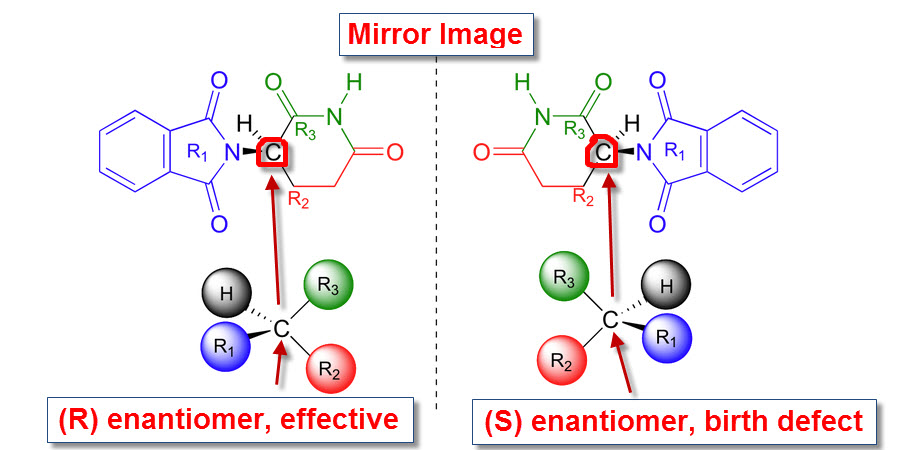
The (R) enantiomer was effective as an anti-nausea drug, but the (S) enantiomer acted as the teratogen (substance that causes malformations of the fetus).
In production of the drug, the manufacturers ignored the extraordinary character of the chemical compound involved and marketed it anyway. In fact, it would have been utterly expensive had they decided to separate the two enantiomers!
After this incident, all pharmaceutical drugs had to undergo rigorous testing prior to their introduction into the marketplace (Kelsey, 1988). In 2012, after nearly 50 years of the tragedy, the inventor of thalidomide, the Grünenthal Group released a statement saying it regrets the consequences of the drug!
Sources:
http://toxsci.oxfordjournals.org/content/122/1/1/F1.expansion.html
http://toxsci.oxfordjournals.org/content/122/1/1.full
http://www.theguardian.com/society/2012/sep/01/thalidomide-scandal-timeline
http://www.rxlist.com/thalomid-drug.htm
http://lawrencekok.blogspot.com/2011/06/ib-chemistry-stereoisomerism-optical.html
