What are mice?

Mice are small rodents and can be identified by their characteristic features. They are small rodents with slender, slightly pointed noses, nearly hairless tails, protruding eyes, and sparsely-haired, large ears. The best-known mouse species is Mus musculus (house mouse). When a muroid rodent is discovered, its common name includes the tern mouse if it is smaller in size, and the term rat is used if it’s bigger. The terms rat and mouse aren’t taxonomically specified.
Typically, the term mice is used for rodents that are classified under the genus Mus but can also include species from other genera, such as Peromyscus (deer mouse).
Why are mice considered a suitable animal model?
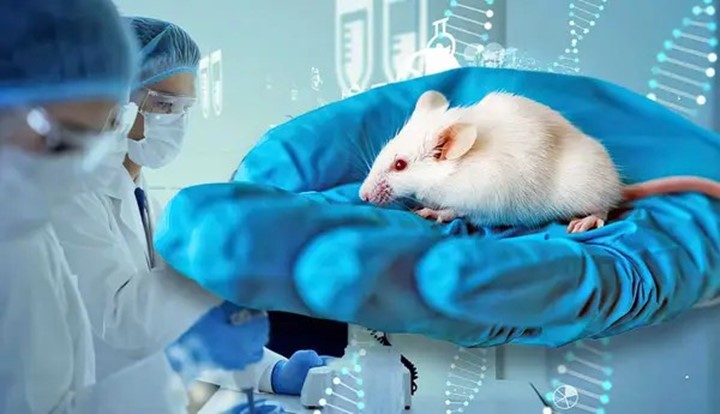
Because of its tiny size and great reproductive habits, the mouse is the most prominent animal model used in human health and disease research. It is also a relatively inexpensive option.
Mice are among the first mammals whose genes we have been able to edit, thanks to more than a century of research into their physiology, anatomy, and genetic makeup. This knowledge has been made possible by the development of molecular tools that allow for gene modification.
Mice are regarded as appropriate animal models for research because of their genetic resemblance to humans, short reproductive cycles, ease of breeding, and comparatively cheap upkeep costs. Their compact size facilitates effective housing and experimentation, and their biological and physiological resemblances to humans render them useful for researching a range of illnesses.
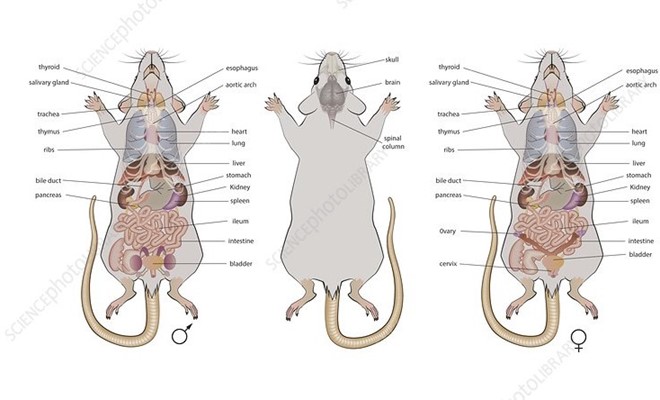
Genetic background of Mice
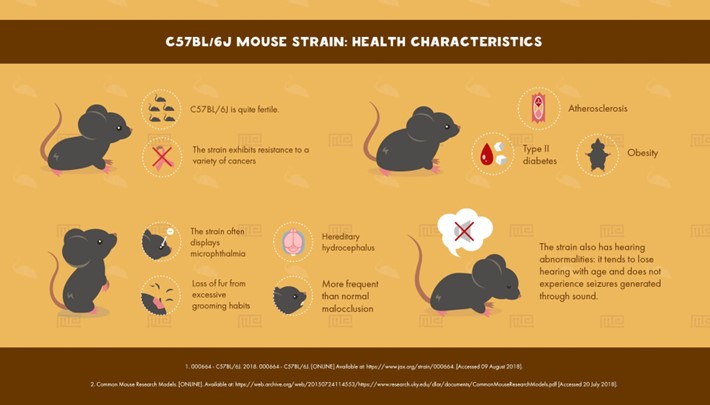
In mouse research, selecting a genetic background is essential to producing accurate and significant findings. In order to maintain genetic homogeneity, scientists had to transition from outbreeding mice, which initially brought a considerable degree of genetic variability, to inbred strains. Still, inbred strains themselves show innate variations in biological characteristics and susceptibility to illness. With more than 450 inbred strains at their disposal, scientists can examine a variety of genotypes and traits with great flexibility. Because of the C57BL/6 strain’s high genetic information and repeatability, it is very well-liked for use in biomedical research.
Although inbred strains have advantages like higher reproducibility and statistical power, outbred stocks reflect the genetic heterogeneity found in the human population and might be better suited for some research concerns. Ultimately, selecting the appropriate genetic background is essential for the success and relevance of the study.
Approaches to modeling human genetic conditions to mice
The development of genetically engineered mouse models has revolutionized research in human genetic conditions over the past 40 years. Initially, conventional transgenesis allowed for the random integration of DNA fragments into the mouse genome, producing transgenic animals.
Later, homologous recombination in embryonic stem (ES) cells enabled precise targeting of specific genes, earning its discoverers the Nobel Prize in Physiology or Medicine in 2007. This method involves replacing or deleting specific gene sequences in ES cells, selecting desired clones, and then introducing them into mouse blastocysts to create chimeric mice. Knockout (KO) mice involve deleting or inactivating endogenous genes, while knockin (KI) mice entail introducing specific modifications into the genome. Advancements like CRISPR/Cas9 technology have further expanded possibilities, allowing for precise gene editing in mouse zygotes and the direct generation of conditional alleles.
As genetics research has evolved from single-gene studies to exploring the entire genome, designing animal models requires consideration of the complexity involved. Different approaches for generating genetic models for specific biological questions are discussed, emphasizing practical examples and personal experiences. Overall, these advancements provide researchers with an array of tools to create precise and relevant models for studying human genetic conditions in mice.
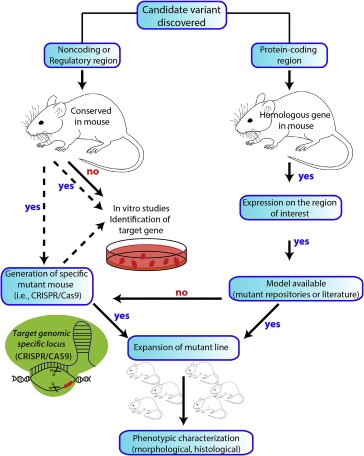
Once a candidate variant, believed to be responsible for a determined phenotype in human pathology, is identified, different research strategies will follow—either the generation of a new genetically modified mouse model or the identification of an existing one—to achieve one of the ultimate goals in biomedical research: the study of pathological human conditions in a model organism in vivo. CRISPR/Cas9, clustered regularly interspaced short palindromic repeats/Cas9 nuclease.
Mouse Models of Human Disease
When delving into mouse models of human disease, two key aspects stand out: the precision of disease induction and the fidelity of mimicking human disease manifestations. These criteria serve as the yardstick for assessing the relevance of any mouse model. Given the vast array of available models, a comprehensive evaluation of each would be arduous. Thus, our focus here is on examining the accuracy of mouse models of human lung diseases such as asthma, chronic obstructive pulmonary disease (COPD), and pulmonary fibrosis. We delve into the mechanisms of disease induction and the models’ ability to replicate crucial aspects of human disease pathology and response to treatment, shedding light on their utility in advancing our understanding of these conditions.
Asthma
In the pursuit of modeling human inflammatory lung diseases like asthma in mice, a crucial consideration lies in acknowledging the disparities between the two species. Take, for instance, the pivotal role of mast cells in human asthma. These immune cells exhibit notable variations in granule composition and airway distribution between mice and humans. While mice predominantly lack peripheral lung mast cells, humans boast a rich population of diverse mast cell subtypes in the alveolar parenchyma. Moreover, anatomical distinctions, such as the less extensive pulmonary circulation in mice compared to humans, can significantly influence leukocyte behavior and inflammation dynamics. However, despite these disparities, leveraging mouse models with a nuanced understanding of these differences can unlock valuable insights into identifying novel therapeutic targets for human disease management.
COPD
Chronic Obstructive Pulmonary Disease (COPD) stands as a formidable challenge in the realm of respiratory ailments, marked by persistent airway obstruction—a feature that distinguishes it from the reversible obstruction observed in asthma. Clinically, COPD encompasses a spectrum of conditions, including chronic bronchitis and emphysema, often triggered by prolonged exposure to tobacco smoke or, in rare instances, genetic factors like α1-antitrypsin deficiency. The pathogenesis of COPD is multifaceted, evolving over years of smoking and influenced by genetic predisposition and environmental factors. Inflammatory processes play a pivotal role in COPD, albeit with a distinct cellular landscape dominated by neutrophils and, to a lesser extent, macrophages. Given the intricate nature of COPD’s etiology, crafting a mouse model that faithfully recapitulates all its facets proves challenging. Instead, researchers typically aim to induce COPD-like lesions by exposing mice to noxious substances—chiefly cigarette smoke—or by administering tissue-degrading enzymes to mimic emphysematous changes. Through these models, scientists strive to unravel the intricate mechanisms underlying COPD pathogenesis and pave the way for innovative therapeutic interventions.
Pulmonary fibrosis
Pulmonary fibrosis, characterized by the abnormal accumulation of fibrotic tissue within the alveolar parenchyma, represents a complex array of diseases with diverse etiologies in humans. Among these, idiopathic interstitial pneumonias, notably idiopathic pulmonary fibrosis (IPF), stand out for their enigmatic nature and grim prognosis. IPF manifests as progressive fibrosis, indicative of flawed tissue regeneration mechanisms, although the underlying cause remains elusive. Suspected to stem from a blend of immunological, genetic, and environmental factors, accurately modeling this disease in mice poses a formidable challenge. While bleomycin administration is a common method used to induce pulmonary fibrosis in both mice and humans, it may not fully capture the multifaceted origins of human pathology. Notably, the choice of mouse strain also plays a pivotal role, with C57BL/6 exhibiting susceptibility to fibrotic changes, while BALB/c displays relative resistance—a reflection of divergent cytokine responses to cellular stress and injury. Whether administered locally or systemically, bleomycin elicits varying responses, underscoring the complexity of modeling pulmonary fibrosis in mice and the ongoing quest to unravel its intricate mechanisms.
Advantages of using the Mouse as a Model Organism
Given their similar genetic backgrounds, mice and humans share many biological traits and are susceptible to many of the same diseases. It is possible to genetically modify mice to resemble almost any human illness or ailment. The specific mutation(s) causing human disease can be implanted into mice using contemporary sequencing and genomic engineering techniques, producing more precise and valuable disease study data.
To create genetically identical strains, mice can be inbred. This consistency makes the experiments more reproducible and accurate. Currently, the Jackson Laboratory keeps almost nine thousand genetically characterized mouse strains.
Mice live longer than humans do; a mouse year is about equivalent to thirty human years. It is therefore possible to study their complete life cycle in just two or three years.
Limitations and challenges of using mice
Although human and mouse diseases are similar, they’re not the same. Findings from the mouse aren’t guaranteed to be the same in people. Some drugs that work in mice don’t work or aren’t safe for humans.
Many animals need to be bred for genetic experiments.
Some of these won’t be used, because they won’t all have the right genetics.
We don’t fully understand what could happen if genetically engineered animals entered the natural ecosystem.
As with all animal research, there are ethical and moral questions to consider.
Written by:
W.R.K. Fernando and Bhagya Babaranda
3rd Year Undergraduates
Faculty of Science,
University of Colombo.
References:
- Advantages of using the Mouse as a Model Organism- Oxford Instruments. (n.d.). Oxford Instruments. https://andor.oxinst.com/learning/view/article/advantages-of-using-the-mouse-as-a-model-organism
- Canales, C. P., & Walz, K. (2019). The Mouse, a model organism for biomedical research. In Elsevier eBooks (pp. 119–140). https://doi.org/10.1016/b978-0-12-816573-7.00006-7
- Model Organism – The Mouse https://www.yourgenome.org/theme/model-organisms-the- mouse/#:~:text=Limitations%20and%20challenges%20of%20using,be%20bred%20for%20genetic%20experim ents.
- The Applicability of Mouse Models to the Study of Human Disease https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7121329/
- Vandamme, T. F. (2014). Use of rodents as models of human diseases. Journal of Pharmacy and Bioallied Sciences, 6(1), 2. https://doi.org/10.4103/0975-7406.124301
- What is a mouse model. (n.d.). Mouse as a model. https://www.jax.org/why-the- mouse/model#:~:text=Mice%20can%20be%20genetically%20manipulated,and%20useful%20disease%20resea rch%20data.
Image Courtesy:
- Title Image: https://bitly.ws/3dPkE
- 1st Content Image: https://bitly.ws/3dPkM
- 2nd Content Image: https://bitly.ws/3dPkU
- 3rd Content Image: https://bitly.ws/3dPm2
- 4th Content Image: https://bitly.ws/3dPm6



0 Comments