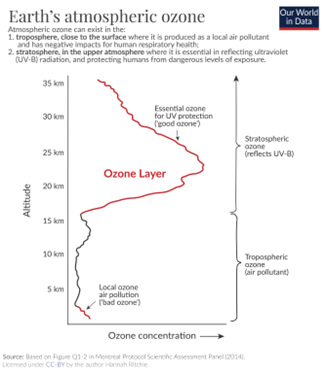
Ozone (O3) is formed through the process of passing dry oxygen (O2) through an electric discharge or spark. This process results in the conversion of oxygen molecules into ozone molecules, with the chemical equation O2 + O → O3. The product obtained from this process is called “ozonized oxygen.” Ozone in the stratosphere is primarily formed through photodissociation, a process where high-energy solar photons break the chemical bonds within oxygen molecules. This results in the release of single oxygen atoms.
These liberated oxygen atoms can then combine with intact oxygen molecules to form ozone. This natural process of ozone formation has been ongoing for billions of years, gradually building up ozone levels in Earth’s atmosphere. The formation of the ozone layer in the stratosphere was instrumental in the development of life on Earth. It shielded the planet from lethal levels of UVB radiation (ultraviolet radiation with wavelengths between 315 and 280 nm), enabling life forms to migrate from the oceans to land.
Over the last few decades, human activities have significantly altered the ozone layer in the stratosphere. Ozone depletion refers to the global decrease in stratospheric ozone observed since the 1970s. This phenomenon is most pronounced in polar regions.
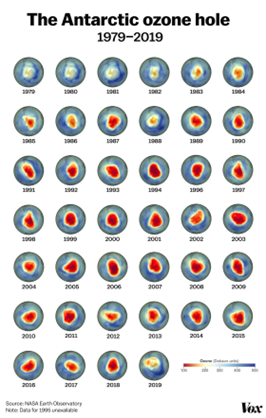
The primary culprits behind ozone depletion are chlorine and bromine compounds in the stratosphere, particularly those released by human-made chemicals like chlorofluorocarbons (CFCs) and other halocarbons. UV radiation from the sun breaks these chlorine and bromine compounds apart, releasing them into the stratosphere. Once freed, chlorine and bromine atoms can destroy ozone molecules by stripping away single oxygen atoms from them. Ozone depletion is so extensive that it leads to the formation of what are commonly known as ozone holes. These are regions with severely reduced ozone coverage. Ozone holes are most prominent over the polar regions, particularly over Antarctica during its spring season. The largest and most well-known ozone hole over Antarctica has spanned more than 20.7 million square km (8 million square miles) consistently since 1992. These holes represent areas where ozone levels have significantly declined, leaving a vulnerable gap in the protective ozone layer.
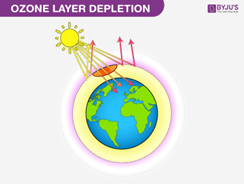
Ozone depletion refers to the reduction in the concentration of ozone in the stratosphere, particularly in the ozone layer. The depletion of the ozone layer has been linked to the presence of chlorofluorocarbon (CFC) compounds in the atmosphere. When CFCs are released into the atmosphere, they eventually reach the stratosphere, where they are exposed to ultraviolet (UV) rays from the sun. Under the influence of UV radiation, CFC molecules are broken down into chlorine radicals, as shown in the equation CF2Cl2 (g) → Cl (g) + CF2Cl (g), where Cl represents a chlorine radical. These chlorine radicals (Cl) then react with ozone (O3) to form chlorine monoxide (ClO) and molecular oxygen (O2), as shown in the equation Cl (g) + O3 (g) → ClO (g) + O2 (g). This reaction involving chlorine radicals and ozone molecules leads to the breakdown of ozone, depleting the protective ozone layer.
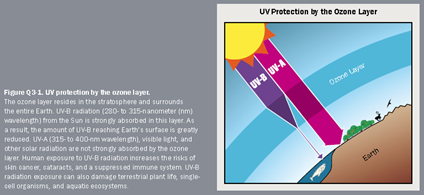
The stratospheric ozone layer acts as Earth’s protective shield, safeguarding living organisms from excessive ultraviolet radiation emitted by the sun. Unfortunately, the release of ozone-depleting substances has inflicted harm upon this vital layer. However, thanks to concerted efforts at both domestic and international levels, the ozone layer is on the path to recovery and is expected to fully heal by approximately 2065. This website is dedicated to addressing stratospheric ozone concerns, encompassing topics such as the science behind ozone depletion, the EPA’s regulatory strategies for ozone layer protection, approved alternatives to ozone-depleting substances, and guidelines for sun safety. Diminished ozone levels due to ozone depletion translate into reduced protection from the sun’s rays, leading to increased exposure to harmful UVB radiation at the Earth’s surface. Research has consistently demonstrated that in the Antarctic region, the quantity of UVB radiation measured at the surface can effectively double during the occurrence of the annual ozone hole. This concerning phenomenon underscores the critical role of the ozone layer in shielding us from the harmful effects of elevated UVB radiation The depletion of the ozone layer leads to an escalation in the influx of UVB (ultraviolet-B) radiation reaching the Earth’s surface. Extensive laboratory and epidemiological research provides compelling evidence that UVB exposure is a primary factor in the development of non-melanoma skin cancer and significantly contributes to the onset of malignant melanoma. Furthermore, UVB radiation has been directly associated with the formation of cataracts, a condition characterized by the clouding of the eye’s lens. These findings underscore the critical importance of preserving the ozone layer as a shield against the adverse health effects associated with increased UVB radiation exposure.
UVB (ultraviolet-B) radiation indeed exerts a significant impact on the physiological and developmental processes of plants. While plants have evolved mechanisms to mitigate or repair the effects of UVB radiation and can adapt to increased levels over time, it’s essential to recognize that UVB radiation can directly influence plant growth. However, it’s not just the direct effects of UVB that matter; indirect consequences also play a crucial role. These indirect changes, which encompass alterations in plant morphology, nutrient distribution within the plant, timing of developmental phases, and secondary metabolism, can often be as, or even more, significant than the direct damage caused by UVB radiation. These secondary effects can have profound implications for various aspects of plant life and ecosystems: Competitive Balance: Changes in plant form and growth patterns can impact their competitive ability within ecosystems. Some plants may be better equipped to cope with increased UVB exposure, giving them a competitive advantage over others. Herbivory, Altered nutrient distribution, and changes in secondary metabolism can affect the nutritional quality of plants. This, in turn, can influence herbivore behaviour and preferences, potentially impacting herbivore-plant interactions. Plant Diseases, UVB-induced changes in plant physiology can affect their susceptibility to diseases. Some plants may become more or less vulnerable to pathogens due to UVB exposure. Biogeochemical Cycles, Changes in plant growth, nutrient cycling, and metabolism can have broader effects on biogeochemical cycles, such as carbon and nitrogen cycling in ecosystems. These changes can impact nutrient availability for other organisms in the ecosystem. Understanding the intricate interplay between UVB radiation and plants is essential for comprehending how these changes cascade through ecosystems. It highlights the interconnectedness of different species and processes within natural environments and underscores the importance of monitoring and mitigating factors contributing to ozone depletion to protect both human and ecological health.
Phytoplankton, the microscopic photosynthetic organisms found in aquatic ecosystems, serve as the foundational building blocks of aquatic food webs. Their productivity is restricted to the euphotic zone, which represents the upper layer of the water column where sufficient sunlight penetrates to support net photosynthesis and growth. However, the impact of solar UVB (ultraviolet-B) radiation on phytoplankton is a growing concern due to its potential to disrupt these essential organisms and, consequently, aquatic ecosystems. Solar UVB radiation has been shown to have adverse effects on phytoplankton. It can alter their orientation and motility, resulting in reduced survival rates. Furthermore, the increased UVB radiation associated with ozone depletion has led to a direct reduction in phytoplankton production. This has far-reaching consequences because phytoplankton form the base of aquatic food chains, and any reduction in their abundance can disrupt the entire ecosystem. The effects of UVB radiation are not limited to phytoplankton; they extend to various marine animals, including fish, shrimp, crabs, amphibians, and other aquatic organisms. These impacts are particularly pronounced during the early developmental stages of these animals. Some of the observed effects include Decreased Reproductive Capacity: UVB radiation can impair the reproductive capacity of marine animals, leading to reduced breeding success. This can result in diminished population sizes over time. Impaired Larval Development, UVB exposure can negatively affect the development of larvae, potentially causing deformities, reduced growth rates, and decreased survival. Population Reductions, Even small increases in UVB exposure can lead to population reductions in small marine organisms. These reductions can have profound implications for the entire marine food chain because many species rely on these organisms as a primary food source. The cumulative impact of UVB-induced damage to phytoplankton and various marine animals can disrupt the delicate balance of aquatic ecosystems. This disruption can affect not only the abundance and diversity of species within these ecosystems but also the overall health and productivity of marine environments. Consequently, the consequences of ozone depletion-related increases in UVB radiation extend beyond the stratosphere and have tangible effects on the intricate web of life in our oceans and other aquatic habitats.
Increases in UVB (ultraviolet-B) radiation, often associated with ozone depletion, have the potential to significantly impact terrestrial and aquatic biogeochemical cycles. These changes can alter both the sources and sinks of various greenhouse and chemically important trace gases, resulting in complex and interconnected effects on Earth’s atmosphere and ecosystems. Here, we delve into the potential consequences of increased UVB radiation on biogeochemical cycles and the subsequent feedback that can either mitigate or amplify the concentrations of key gases. Carbon Dioxide (CO2), and UVB radiation can affect the photosynthetic activity of plants, including terrestrial vegetation and phytoplankton in aquatic ecosystems. Changes in plant productivity can influence the uptake and release of carbon dioxide. Increased UVB exposure may alter carbon cycling, potentially affecting atmospheric CO2 concentrations. This, in turn, can contribute to feedback mechanisms influencing global climate. Carbon Monoxide (CO), and UVB radiation can influence the production and decomposition of organic matter in terrestrial and aquatic environments. Carbon monoxide is a byproduct of organic matter decomposition. Changes in UVB exposure may impact the rates of CO production and consumption, potentially affecting atmospheric CO levels. Carbonyl Sulfide (COS), Carbonyl sulphide is involved in various biogeochemical processes, including plant stomatal uptake. Increased UVB radiation can influence COS dynamics by affecting plant physiology and stomatal conductance. Changes in COS concentrations can have implications for the sulphur cycle and atmospheric chemistry. Ozone (O3), While ozone depletion in the stratosphere contributes to increased UVB at the Earth’s surface, it can also influence tropospheric ozone dynamics. UVB radiation is involved in the photochemical production of ozone in the lower atmosphere. Changes in UVB levels can affect tropospheric ozone concentrations, which play a role in air quality and climate-related processes. Other Gases and UVB radiation may also impact the production and destruction of other trace gases, including volatile organic compounds (VOCs) and nitrogen oxides (NOx). These gases are involved in atmospheric chemistry, affecting air quality and climate. Biosphere-Atmosphere Feedbacks, The changes in the sources and sinks of these gases due to increased UVB radiation can trigger biosphere-atmosphere feedbacks. This feedback can either amplify or mitigate the concentrations of these gases in the atmosphere, leading to various consequences: Positive Feedback, For example, if increased UVB radiation leads to reduced plant productivity, resulting in decreased CO2 uptake, it can amplify atmospheric CO2 concentrations, contributing to global warming. Conversely, if increased UVB radiation stimulates the decomposition of organic matter and accelerates carbon cycling, it may enhance carbon sequestration, mitigating the rise in atmospheric CO2 levels.
UVB radiation has a significant impact on various materials, including synthetic polymers, naturally occurring biopolymers, and other materials of commercial interest. These materials are often used in outdoor applications and are vulnerable to UVB-induced degradation. While current technologies incorporate special additives to provide some degree of UVB protection, the anticipated increases in UVB radiation due to factors like ozone depletion can accelerate the breakdown of these materials, thus reducing their outdoor durability. Here’s a closer look at how UVB radiation affects these materials and the implications for their outdoor use. Synthetic Polymers, Synthetic polymers, such as plastics and synthetic rubbers, are widely used in outdoor applications ranging from construction materials to automotive components and consumer goods. UVB radiation can cause polymer chains to break or undergo chemical changes, leading to a range of detrimental effects. This includes surface degradation, loss of mechanical strength, colour fading, and increased brittleness. Special UV stabilizers and additives are incorporated into polymer formulations to mitigate these effects. However, as UVB levels increase, these protective additives may become less effective, shortening the useful lifespan of polymer-based products exposed to sunlight. Naturally Occurring Biopolymers, Natural biopolymers, such as cellulose (found in wood) and proteins (e.g., silk), are also susceptible to UVB-induced damage. Exposure to UVB radiation can result in the degradation of these materials, leading to changes in their mechanical properties, colour, and texture. In outdoor environments, biopolymers like wood can undergo photodegradation, which includes surface cracking, discoloration, and a decrease in structural integrity. Protective coatings and treatments can help extend the durability of these materials, but increased UVB levels can challenge these preservation efforts. Other Materials of Commercial Interest, Beyond polymers and biopolymers, various materials used in commercial applications, such as paints, coatings, textiles, and adhesives, can also be adversely affected by UVB radiation. For instance, UVB exposure can lead to paint and coating deterioration, colour fading, and loss of adhesion. In textiles, UVB can cause fabric weakening and fading of dyes. The performance of these materials often relies on UV-absorbing or UV-blocking additives, which may require optimization or replacement to withstand higher UVB levels. The production and depletion of ozone in the stratosphere are pivotal processes that have shaped Earth’s atmosphere and influenced the development of life on our planet. Let’s explore these processes in more detail:
Ozone Depletion and Human Activities, Over the last few decades, human activities have significantly altered the ozone layer in the stratosphere. Ozone depletion refers to the global decrease in stratospheric ozone observed since the 1970s. This phenomenon is most pronounced in polar regions. The primary culprits behind ozone depletion are chlorine and bromine compounds in the stratosphere, particularly those released by human-made chemicals like chlorofluorocarbons (CFCs) and other halocarbons. UV radiation from the sun breaks these chlorine and bromine compounds apart, releasing them into the stratosphere. Once freed, chlorine and bromine atoms can destroy ozone molecules by stripping away single oxygen atoms from them.
Formation of Ozone Holes, Ozone depletion is so extensive that it leads to the formation of what are commonly known as ozone holes. These are regions with severely reduced ozone coverage. Ozone holes are most prominent over the polar regions, particularly over Antarctica during its spring season. The largest and most well-known ozone hole over Antarctica has spanned more than 20.7 million square km (8 million square miles) consistently since 1992.
These holes represent areas where ozone levels have significantly declined, leaving a vulnerable gap in the protective ozone layer.
Ozone Day serves as a vital reminder of the significance of our planet’s ozone layer and the imperative to safeguard it. This protective shield in the stratosphere, composed of ozone molecules, shields life on Earth from the harmful effects of ultraviolet (UV) radiation from the sun.
We have a responsibility to protect our planet from harmful gases and safeguard the ozone layer.
Written by:
Keertheka Yuvarajah
4th year undergraduate
Horticulture and Sustainable Landscaping
Faculty of Science,
University of Colombo.
References:
- de Gruijl FR, van der Leun JC. (2000) Environment and health: 3. Ozone depletion and ultraviolet radiation. CMAJ. https://byjus.com/chemistry/ozone-layer/
- The ozone layer – DCCEEW. (2021, October 9). https://www.dcceew.gov.au/environment/protection/ozone/ozone-science/ozone-layer
- Health and environmental effects of ozone layer depletion | US EPA. (2023, September 11). US EPA. https://www.epa.gov/ozone-layer-protection/health-and-environmental-effects-ozone-layer-depletion
- Admin. (2022). Ozone Layer Depletion – Cause, Effects, And Solutions. BYJUS. https://byjus.com/biology/ozone-layer-depletion/
- Piper, K. (2023, January 10). The ozone hole shrank, showing the world can solve environmental crises. Vox. https://www.vox.com/future-perfect/22686105/future-of-life-ozone-hole-environmental-crisis-united-nations-cfcs
- What is the ozone layer, and why is it important? (2023, March 13). Our World in Data. https://ourworldindata.org/ozone-layer-context
- Rinkesh. (2023, July 31). Ozone Layer and Causes, effects and solutions to ozone depletion – Conserve Energy future. Conserve Energy Future. https://www.conserve-energy-future.com/ozone-layer-and-causes-of-ozone-depletion.php
- McKenzie, R. L., Bernhard, G., Liley, B., Disterhoft, P., Rhodes, S., Bais, A. F., Morgenstern, O., Newman, P. A., Oman, L. D., Brogniez, C., & Simic, S. (2019). Success of Montreal Protocol Demonstrated by Comparing High-Quality UV Measurements with “World Avoided” Calculations from Two Chemistry-Climate Models. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-48625-z
Image Courtesy:
- Title Image: https://bit.ly/48lyB2P
- 1st Content Image: https://biturl.top/nai2y2
- 2nd Content Image: https://bit.ly/3EDiXlY
- 3rd Content Image: https://biturl.top/qUn2aq
- 4th Content Image: https://biturl.top/jaE7Bv
- 5th Content Image: http://bit.ly/3rjZwLR



0 Comments