“Studying C. elegans is like peering through a microscope to the inner workings of life”
-Robert Horvitz(An American Biologist)
In the vast fields of Biology and the tapestry of life, there is a superhero model organism that has immensely aided scientists and researchers “to see the unseen” for the past several decades. Being called “the lab mascot,” “the living microscope,” or “the transparent wonder,” this superhero is none other than Caenorhabditis elegans, shortly referred to as C. elegans. This tiny nematode worm rules the laboratories in genetics, developmental biology, ageing, neurobiology, epigenetics, and other human diseases, securing 3 Nobel prizes. Despite being a tiny worm, this remarkable creature hosts many desirable and mysterious secrets to reveal the untold, underlined truths of life from different perspectives through ground-breaking, cutting-edge research. Therefore, join hands with us to deep dive into the tiny mysteries of this tiny superstar, in the labs.
C. elegans: The mysterious fellow in the lab
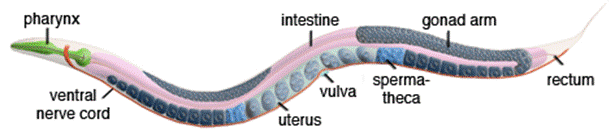
C. elegans (Caenorhabditis elegans) is a free-living, transparent, and mostly hermaphroditic (i.e., possessing both sexes in the same animal) worm, approximately 1mm in length, living in temperate soil environments. Though it possesses a simple body plan compared to a mammal, one should not be deceived by its size and simplicity, which underline much more complex cellular, physiological, and genetic processes taking place in this tiny giant. Despite its small body size, it boasts the complexity of possessing a well-developed digestive system, nervous system, reproductive system, etc. for it to be an ideal model organism in medical and genetic research.
This remarkable organism was first studied in the laboratory by the scientists: Victor Nigon and Ellsworth Dougherty and was first used as a model organism by Sydney Brenner in 1936, to study the genetic basis of developmental biology. Though it started with developmental biology, the applications of C. elegans have expanded into cutting-edge research in gene expression, studies on cellular processes such as cell division and cell death, neurobiology, ageing, drug testing, genetics, modelling of human diseases, etc, proving the versatility of this mysterious creature as a model organism. Cutting-edge research with C. elegans has granted 6 scientists 3 Nobel Prizes for studies on cell death, RNAi-mediated gene expression regulation, and the discovery of Green Fluorescent Protein (GFP), demarcating its immense contribution towards modern science. Therefore, as Sydney Brenner once mentioned, if a Nobel prize is given to the model organism, this tiny, but mysterious creature will undoubtedly be the perfect candidate.

C. elegans: Being the ideal model organism
Many attributes of this fascinating creature have allowed it to be a versatile model organism in modern scientific research. First, It is easy to culture due to them being able to survive both in the laboratory and in natural conditions with the same diet as E, coli. Secondly, the fast growth of C. elegance being able to develop into an adult of 1.3mm from the egg within 3 days serves as a desirable factor. Moreover, the small body size allows the assays involving C. elegance to be performed in well-plates, which eliminates the cumbersome experience of handling larger animals as models. The transparent nature of C. elegance allows its use in fluorescence-related research, and its huge anatomical and physiological complexity makes it an ideal model organism. Other than that, shared developmental, genetic, neurological, and cellular features with humans make this fascinating fellow a good model for genetic, drug screening, neurological, etc. studies.
C. elegans: The Genetic Goldmine in Modern Research
This mysterious fellow in the lab acquired the opportunity to be the first multicellular organism to get its entire genome sequenced. Possessing a small and simple genome of six chromosomes, with approximately 100 000 000 base-pairs, has made them ideal for genetic manipulation, compared to other more complex mammalian models. Apart from that, their large number of progeny, rapid life cycle, and dual mode of reproduction also assist in C. elegance becoming truly a genetic goldmine.
Moreover, C. elegans conserves genetic regions with humans, making it a suitable model for genetic and epigenetic research. Such conserved genes include the genes that are responsible for the development of C. elegans, genes involved in signalling pathways, including insulin-related pathways, and genes involved in DNA repair, nerve function, and immunity in C. elegans. These resemblances not only allow C. elegans to be a genetic model but also to unravel its potential as a neurological, developmental, drug-screening, and immunity-related model organism.
C. elegans: Genetic Tools and Forward Genetics
C. elegans, a diploid organism with six chromosomes, including five autosomes and one sex chromosome, is commonly used in genetic research due to its rapid lifecycle and ease of genetic manipulation. Classical forward genetic screens involve mutagenizing the nematode germ line, isolating heterozygous mutants, and self-fertilizing to obtain homozygous mutants. Mutants with visible phenotypes, such as Unc (uncoordinated movement) or Dpy (dumpy-shaped animals), are easily identified using microscopy. Various mapping strategies, including crosses with marker strains and whole genome sequencing, are employed to identify the causative mutations.
In addition to forward genetic screens, modifier screens are used where animals with a known phenotype are mutagenized to isolate suppressor or enhancer mutations. These modifiers can be genetically separated from the original mutation to study their individual phenotypic effects. The rapid genetic crosses in C. elegans facilitate epistasis studies to understand novel mutations within known genetic pathways. Overall, the combination of rapid lifecycles, visible phenotypes, and efficient genetic manipulation makes C. elegans a powerful model organism for genetic research.
C. elegans: Identifying Novel Human Disease Genes
Innate immunity
Nowadays, C. elegans is used as a model organism to study the innate immune response to pathogens. It has become a significant model system for the identification of innate immune genes. It lacks migratory immune cells and does not have an adaptive immune response. C. elegans relies on an innate immune response, involving the production of antimicrobial peptides and compounds to fight infections.
Neuroscience
The nervous system of C. elegans is extensively mapped, providing a robust model for studying neural specification, development, function, and connectome analysis. C. elegans nervous system is used as a model for the cellular basis of human neural diseases and disorders, including an increased focus on the physiology and function of glia in C. elegans. Due to the worm’s relatively small number of neurons and well-known cell connections, computer modelling of the worm’s connectome is a valuable tool for defining minimal circuits and simulating complex behaviours.
Nobel prize-winning experiments that used C. elegans to explore the nervous system, its development, and function, helped to understand brain physiology. The effects of many toxic substances (pesticides, metals) have been studied in the nervous system of C. elegans. The results of these studies explained similar effects of chemicals that are often seen in mammalian systems.
Ageing
As we grow older, we become increasingly frail and eventually die. These include almost all of the major neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, cardiovascular disease, and cancer. However, our understanding of ageing has been transformed by pioneering studies in C. elegans over the last 25 years. A relatively short lifespan of 20 days makes C. elegans an excellent system to study ageing. Its short lifespan makes it possible to conduct experiments. These studies showed that ageing is a regulated phenomenon that can be studied with the tools of molecular biology and that many of the genes that regulate ageing in nematodes also regulate ageing in other organisms. By genetically manipulating the worms to be less susceptible to illness, scientists can identify the underlying factors influencing ageing.

C. elegans: Modeling Human Diseases
Several genomic and candidate gene-based RNAi screens have been performed in C. elegans models of Parkinson’s disease. These models have mainly focused on the overexpression of α-Synuclein, a key candidate Parkinson’s disease protein. Although α-Synuclein is not naturally present in C. elegans, researchers have overcome this by introducing human α-Synuclein into the nematodes to screen for genes influencing α-Synuclein aggregation or cell function.
Alzheimer’s disease, the sixth leading cause of death in the USA, affects over 5 million people in the USA and more than 35 million worldwide. With the ageing population, the incidence of Alzheimer’s is expected to increase. Despite intensive research, many aspects of the disease remain a mystery, and effective treatments have not been developed. Research efforts often focus on understanding the aggregation of proteins like tau or beta-amyloid and their impact on neurological function.
In C. elegans models, researchers have utilized the overexpression of wild-type or mutant Tau to simulate tauopathy. Expression of Tau in all nematode neurons led to Tau aggregation and a moderate uncoordinated phenotype. A genome-wide RNAi screen was conducted to identify enhancers of this phenotype, revealing genes and pathways similar to those identified in Drosophila screens. The conserved regulators identified in these screens may play a role in tauopathies and Alzheimer’s disease.
C. elegans: Experimental techniques
One of the ultimate advantages of C. elegans as a model organism is the ease of genetic manipulation and the wide array of genetic tools available. C. elegans has 6 chromosomes and approximately 20,000 genes. The C. elegans genome was the first genome of a multi-cellular organism to be completely sequenced. About 40–50% of protein-coding genes have orthologs in humans. It is estimated that 60–80% of human disease-causing genes have orthologs in C. elegans.
RNA interference (RNAi) is an evolutionarily conserved pathway used by multiple species as a mechanism of genome surveillance and antiviral defence. Since its discovery, RNA interference (RNAi) has become an important tool for C. elegans research and the scientific community in general. RNAi is a simple method for determining the loss-of-function phenotypes of genes in C. elegans. RNAi experiments can be performed on worms using several different methods. The original studies of RNAi were performed by injecting dsRNA into the gonad or the body cavity of the worms and examining the phenotypes in the next generation. Lisa Timmons and Andy Fire introduced a technique for feeding worms bacteria expressing dsRNA, and Tabara and Mello showed that simply soaking worms in dsRNA is also effective in producing knock-down phenotypes method. With the introduction of double-stranded RNA into worms, specific inactivation of the corresponding gene occurs through the degradation of endogenous mRNA.
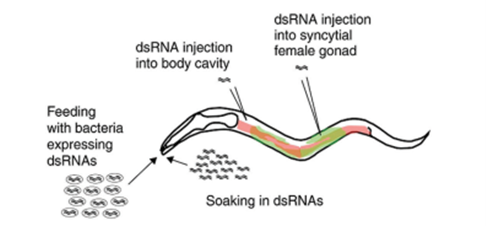
In most organisms, the effects of RNAi are transient and seen only in the first generation. But in C. elegans RNAi effects can persist for several generations. CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats-associated) is the current choice for genome editing. The CRISPR-Cas9 system can be used to target virtually any genomic locus through a guide RNA that recognizes the target DNA via Watson-Crick base pairing.
The first demonstration of CRISPR-Cas9 in C. elegans involved the simple generation of loss-of-function mutants via a non-homologous end-joining (NHEJ) pathway. Several genes play an important role in fertility and development. The disruption of genes leads to arrested growth or sterility. Genetic balancers are chromosomal rearrangements that allow sterile mutations to be stably maintained and have been widely used to study essential genes in organisms. A team of researchers has found an easy way to generate and maintain loss-of-function alleles of essential genes using CRISPR-Cas9 technology. They developed a method to induce chromosomal translocations and produce genetic balancers using CRISPR and used this approach to edit essential genes in C. elegans.
Currently, an increasing number of techniques have been developed to support research on the C. elegans model. Modern technologies, such as artificial intelligence, machine learning, and computational techniques, have been applied to simulate the body structure, nervous system, and behaviours of C. elegans. “OpenWorm” is one of the first projects to simulate C. elegans at the cellular level. The long-term goal of the project is to model all 959 cells of C. elegans.
Fluorescent microscopy is a dynamically evolving field and a key element of C. elegans research. It is employed to study various aspects of C. elegans, such as gene expression, protein localization, and cell dynamics. Fluorescent markers (e.g. green fluorescent protein, GFP) can help us to visualise a protein of interest, giving us clues about what it does, or can label particular neurons. We can see the internal structures of this species due to the transparent outer cuticle with the help of a basic light microscope. DIC microscopy is a widely used technique for imaging C. elegans. It enhances contrast by detecting variations in refractive index, allowing for detailed visualization of the worm’s anatomy and movement. Light sheet microscopy is a technique that uses a thin sheet of light to illuminate the specimen. It enables fast, high-resolution imaging of C. elegans embryos and larvae, minimizing phototoxicity and photobleaching.
C. elegans: Future Directions in Research
Wild isolates of C. elegans have been studied to understand their evolution, development, and biology, including differences in microbiomes compared to laboratory strains. Wild C. elegans strains have low genetic variability and evidence suggests most animals are the offspring of hermaphrodite self-fertilization. They exhibit measurable phenotypic differences, such as longevity, frequency of males, chemo sensation, body size, and sensitivity to toxins/pheromones.
The nervous system of C. elegans is well-mapped, making it a valuable model for studying neural specification, development, function, and connectome analysis. We can understand the cellular mechanisms of learning and memory because of their small nervous system (302 neurons) and well-studied genetics. Computer modelling of the worm connectome is used to define minimal circuits and model complex behaviours, while also being used as a model for understanding human neural diseases and disorders. They are commonly used to study the effects of environmental stress and measure various outcomes such as longevity and development; different forms of stress, including heat shock and oxidative stress, have been identified.
C. elegans shares the nematode phylum with several parasitic roundworms making it a straightforward model to be used for the development of anthelmintic drugs. It is a powerful in vivo model for the development of targeted therapeutics. Features that confer C. elegans an extra value as a model for age‐related proteinopathies research include, among others: (i) the remarkable similarities at the molecular and cellular levels between nematode and vertebrate neurons, (ii) the continuous design of mutant and transgenic C. elegans models of human neurodegenerative disease (iii) the thoroughly studied whole‐animal neuronal connectome which can be individually visualized using fluorescent reporters in the living transparent worm (iv) its short and genetically tractable lifespan.
As we reflect on the journey through this blog post, it becomes evident that C. elegans has emerged not just as a model organism but as a catalyst for innovation in various scientific disciplines. Its contributions to ageing research, neurobiology, and drug discovery underline its versatility and significance. As technology advances and our understanding deepens, this nematode continues to be a beacon of discovery in the scientific world.
Written by:
P.K.D. Chathumini Yasara and Harripriya Sivarathan,
3rd Year Undergraduates,
Immunology and Integrative Molecular Biology Honours,
Faculty of Science,
University of Colombo.
References:
- Kaletta, T., & Hengartner, M. O. (2006). Finding function in novel targets: C. elegans as a model organism. Nature Reviews Drug Discovery, 5(5), 387–399. https://doi.org/10.1038/nrd2031
- Kimble, J., & Nüsslein‐Volhard, C. (2022). The great small organisms of developmental genetics: are Caenorhabditis elegans and Drosophila melanogaster. Developmental Biology, 485, 93–122. https://doi.org/10.1016/j.ydbio.2022.02.013
- Riddle, D. L. (1997). The biological model. C. Elegans II – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK20086/
- About our worms. (n.d.). https://www2.mrc-lmb.cam.ac.uk/groups/worms/history-of-c-elegans-in-scientific-research/
- News-Medical. (2020, May 27). C. elegans as a Model Organism.
- https://www.news-medical.net/life-sciences/C-elegans-as-a-Model Organism.aspx#:~:text=elegans%20has%20been%20used%20as,as%20studying%20the%2immune%20system.
- Xu, Y., & Park, Y. (2018). Application of Caenorhabditis elegans for Research on Endoplasmic Reticulum Stress. Journal of Food Science and Nutrition, 23(4), 275–281. https://doi.org/10.3746/pnf.2018.23.4.275
- Advantages of using Caenorhabditis Elegans as a Model Organism- Oxford Instruments. (n.d.). Oxford Instruments. https://andor.oxinst.com/learning/view/article/advantages-of-using-caenorhabditis-elegans-as-a-model-organism
- Meneely, P., Dahlberg, C. L., & Rose, J. K. (2019). Working with Worms: Caenorhabditis elegans as a Model Organism. Current Protocols Essential Laboratory Techniques, 19(1). https://doi.org/10.1002/cpet.35
Image Courtesy:
- Title Image: https://bitly.ws/3dNjL
- 2nd Content Image: https://bitly.ws/3dNk2
- 3rd Content Image: https://bitly.ws/3dNk6
- 4th Content Image: https://bitly.ws/3dNka
- 5th Content Image: https://bitly.ws/3dNkf
- 6th Content Image: https://bitly.ws/3dNk



0 Comments