Graphite is known as one of the most useful yet underrated elements. It is a crystalline form of the element carbon. What makes it so special is that it has so many uses and also it consists of Nanoparticles.
The earliest use of graphite dates to primitive man, who used it to draw on cave walls. It was also used by Egyptians to decorate pottery. Names like plumbago and black lead have been used to describe the mineral and over time, the term graphite (meaning “to write”) was finally used to describe this mineral.
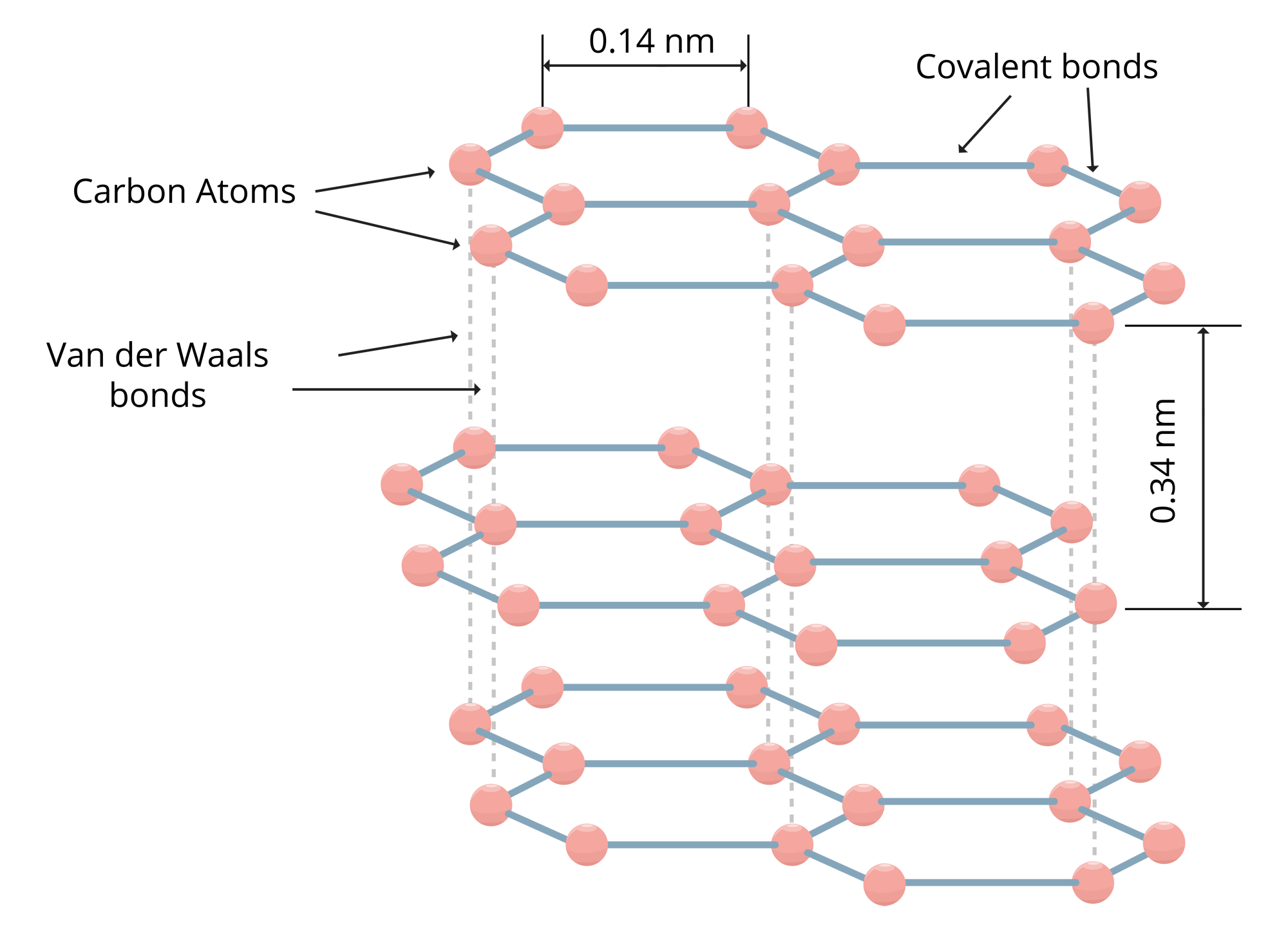
The structure of the graphite is known as the Crystalline Structure. Rings of six carbon atoms arranged in widely spaced horizontal layers. Horizontal bonds are covalent bonds and vertical bonds are Wander Vaal’s. Three out of four atoms of carbon are bounded so the extra electron is free to wander around the layer.
Mainly there are two types of graphite. One type is known as Vein and the other one is called Flake. Among these two types, Flake Graphite covers the most needs of the market since it is easier and cheaper to produce. Flake graphite is typically formed as flat-plate–like particles. Flake needs to be mechanically/chemically processed or “upgraded” to reach the necessary carbon levels for industrial applications. Purity is very low.
On the Other hand, Vein is one of the highest degrees of crystallinity and in some formations, the purest form of the natural crystalline graphite found. As the name suggests, vein graphite is a true vein mineral as opposed to a seam mineral (amorphous graphite) or a mineral that is disseminated throughout the ore rock (as in flake graphite). As Sri Lanka is the only area to find it is known as Ceylon graphite. Below you can see some types of graphite we can find in Sri Lanka. Star granite is said to be the most rare formation.

Bogala mine is well known as a place where graphite is mined in commercial quantities.

When it comes down to the characteristics of graphite, It has high resistance towards high temperatures and chemicals, so we can use it for chemical processing, pharmaceutical and other process applications. Since the electric and thermal conductivity is very high, we can use it as a heat-dissipating or heat-transfer material
And, it is frictionless and has a slippery nature, as a result of its layers structure that I mentioned above.
Although it’s quite difficult to compile, graphite makes our life easier in many ways.
We mainly use graphite for pencils, lubricants, crucibles, foundry facings, polishes, arc lamps, batteries, brushes for electric motors, and cores of nuclear reactors. And also currently some research is going on whether we can use graphite to make Wires, which carry electricity.
So with such immense uses and with significant characteristics of the graphite, which is hidden inside the ground …
Why cannot we call it “The hidden treasure”?
Written by: Aurelia Perera
Image Courtesies:
- Featured Image: http://bitly.ws/LrBd
- Image 1: http://bitly.ws/LrBu
- Image 2: http://bitly.ws/PJ5J
- Image 3: http://bitly.ws/PJ6e
- Image 4: http://bitly.ws/PJ6C
References: